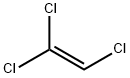
트라이클로로에틸렌
|
|
트라이클로로에틸렌 속성
- 녹는점
- -86 °C
- 끓는 점
- 87 °C
- 밀도
- 1.463 g/mL at 25 °C(lit.)
- 증기 밀도
- 4.5 (vs air)
- 증기압
- 61 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.476(lit.)
- 인화점
- 90°C
- 저장 조건
- 2-8°C
- 용해도
- Soluble in acetone, ethanol, chloroform, ether (U.S. EPA, 1985), and other organic solvents including bromoform, carbon tetrachloride, methylene chloride, trichloroethylene, and tetrachloroethylene.
- 물리적 상태
- 액체
- 색상
- 무색의
- 냄새
- 클로로포름과 에테르의 냄새
- Odor Threshold
- 3.9ppm
- 수용성
- 약간 용해됨. 0.11g/100mL
- Merck
- 14,9639
- BRN
- 1736782
- Henry's Law Constant
- 3.14 at 1.8 °C, 8.47 at 21.6 °C, 19.0 at 40.0 °C, 26.5 at 50 °C, 35.8 at 60 °C, 56.6 at 70 °C (EPICS-GC, Shimotori and Arnold, 2003)
- 노출 한도
- TLV-TWA 50 ppm (~270 mg/m3) (ACGIH), 100 ppm (MSHA and OSHA); TLV-STEL 200 ppm (ACGIH); ceiling 200 ppm (OSHA); carcinogenicity: Animal Lim ited Evidence, Human Inadequate Evidence (IARC).
- Dielectric constant
- 3.4(16℃)
- 안정성
- 안정적인. 산화제, 알루미늄, 마그네슘, 강염기, 환원제와 호환되지 않습니다. 감광성. 많은 금속, 오존, 질산 칼륨, 수산화 칼륨, 수산화 나트륨과 격렬하게 반응합니다.
- LogP
- 2.53 at 20℃
- CAS 데이터베이스
- 79-01-6(CAS DataBase Reference)
- IARC
- 1 (Vol. Sup 7, 63, 106) 2014
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-36/38-52/53-67-68-39/23/24/25-23/24/25-11 | ||
| 안전지침서 | 53-45-61-36/37-16-7 | ||
| 유엔번호(UN No.) | UN 1710 6.1/PG 3 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | KX4550000 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | III | ||
| HS 번호 | 29032200 | ||
| 유해 물질 데이터 | 79-01-6(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 4.92 ml/kg; LC (4 hrs) in rats: 8000 ppm (Smyth) | ||
| IDLA | 1,000 ppm | ||
| 기존화학 물질 | KE-13680 | ||
| 유해화학물질 필터링 | 97-1-309;06-5-11 | ||
| 중점관리물질 필터링 | 별표1-24 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 트리클로로에틸렌 및 이를 85% 이상 함유한 혼합물 |
트라이클로로에틸렌 C화학적 특성, 용도, 생산
개요
에틸렌의 수소 원자 세개가 염소 원자 세개로 바뀐 유기 화합물이다. 무색의 액체로 유독하며 불에 타지 않는다. 휘발성이 있고 달콤한 냄새가 난다.용도
흡입용 전신마취제로 오랫동안 사용되었다.개요
Trichloroethylene (IUPAC), CHClCCl2, is a stable, low-boiling, colorless liquid with a chloroform-like odor. It is not corrosive to the common metals even in the presence of moisture. It is slightly soluble in water and is nonflammable. It is toxic by inhalation, with a TLV of 50 ppm and an IDLH of 1000 ppm in air. The FDA has prohibited its use in foods, drugs, and cosmetics. The four-digit UN identification number is 1710. The NFPA 704 designation is health 2, flammability 1, and reactivity 0. Its primary uses are in metal degreasing, dry cleaning, as a refrigerant and fumigant, and for drying electronic parts.화학적 성질
Trichloroethylene, a colorless (often dyed blue), nonflammable, noncorrosive liquid that has the “sweet” odor characteristic of some chlorinated hydrocarbons. The Odor Threshold is 25-50 ppm.물리적 성질
Clear, colorless, watery-liquid with a chloroform-like odor. Odor threshold concentrations determined in air were 21.4 ppmv (Leonardos et al., 1969) and 3.9 ppmv (Nagata and Takeuchi, 1990). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 10 and 2.6 mg/L, respectively (Alexander et al., 1982).용도
Trichloroethylene is used as a solvent, in drycleaning, in degreasing, and in limited use asa surgical anesthetic.생산 방법
TCE has been in commercial use for almost 60 years. TCE has been used as a solvent because of its powerful ability to dissolve fats, greases, and waxes. It has been widely used in the dry cleaning industry and as a metal degreaser and in the electronic components industry where workers have been observed using it as a cleaning solvent without any protective equipment, thus allowing uncontrolled skin contact and inhalation exposures.정의
ChEBI: A member of the class of chloroethenes that is ethene substituted by chloro groups at positions 1, 1 and 2.일반 설명
A clear colorless volatile liquid having a chloroform-like odor. Denser than water and is slightly soluble in water. Noncombustible. Used as a solvent, fumigant, in the manufacture of other chemicals, and for many other uses.공기와 물의 반응
Slightly soluble in water.반응 프로필
Trichloroethylene has been determined experimentally that mixtures of finely divided barium metal and a number of halogenated hydrocarbons possess an explosive capability. Specifically, impact sensitivity tests have shown that granular barium in contact with monofluorotrichloromethane, trichlorotrifluoroethane, carbon tetrachloride, Trichloroethylene, or tetrachloroethylene can detonate (ASESB Pot. Incid. 39. 1968; Chem. Eng. News 46(9):38. 1968). Trichloroethylene has been determined experimentally that a mixture of beryllium powder with carbon tetrachloride or with Trichloroethylene will flash or spark on heavy impact (ASESB Pot. Incid. 39. 1968). A mixture of powdered magnesium with Trichloroethylene or with carbon tetrachloride will flash or spark under heavy impact (ASESB Pot. Incid, 39. 1968).건강위험
The toxic effects manifested in humansfrom inhaling trichloroethylene vapors areheadache, dizziness, drowsiness, fatigue, andvisual disturbances. A 2-hour exposure to a1000-ppm concentration affected the visualperception. Higher concentrations can pro duce narcotic effects. Heavy exposures maycause death due to respiratory failure or car diac arrest. A 4-hour exposure to 8000 ppmwas lethal to rats. Chronic exposure causedincrease in kidney and liver weights in testanimals.The symptoms of poisoning from oralintake of trichloroethylene are nausea, vom iting, diarrhea, and gastric disturbances. Theacute oral toxicity, however, is low. Theoral LD50 value in mice is in the range2500 mg/kg. Trichloroethylene metabolizesto trichloroacetic acid, which is excreted inthe urine.
Although trichloroethylene exhibits lowtoxicity, its metabolite trichloroethanol, andoxidative degradation products phosgene,COCl2, and chlorine, can cause severe unex pected health hazards. Kawakami andassociates (1988) reported a case of Steven–Johnson syndrome in a worker in a printingfactory. In another case, fire on a stove in ametal-degreasing workplace produced phos gene and chlorine inhalation, which causeddyspnea, fever, and fatigue.
Trichloroethylene exhibited evidence ofcarcinogenicity in laboratory animals. Oraladministration produced liver tumors, whileinhalation caused lung and blood tumors inmice and rats.
화재위험
Special Hazards of Combustion Products: Toxic and irritating gases are produced in fire situations.화학 반응
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.색상 색인 번호
Trichloroethylene is a chlorinated hydrocarbon used as a detergent or solvent for metals, oils, resins, sulfur, and as general degreasing agent. It can cause irritant contact dermatitis, generalized exanthema, Stevens-Johnson- like syndrome, pustular or bullous eruption, scleroderma, as well as neurological and hepatic disorders.잠재적 노출
Trichloroethylene is used as a vapor degreaser of metal parts, as a solvent; and as a drug; It is also used for extracting caffeine from coffee, as a dry-cleaning agent; and as a chemical intermediate in the production of pesticides; in making waxes, gums, resins, tars, paints, varnishes, and specific chemicals; such as chloroacetic acid.Carcinogenicity
Trichloroethylene is reasonably anticipated to be a human carcinogen based on limited evidence of carcinogenicity from studies in humans, sufficient evidence of carcinogenicity from studies in experimental animals, and information from studies on mechanisms of carcinogenesis.신진 대사 경로
From the photooxidation reaction medium (1) of trichloroethylene, the formation of dichloroacetyl chloride, CO, phosgene, and pentachloroethane and their conversion to the final product, CO2, are identified. By the second TiO2 photocatalyst reaction (2), trichloroacetaldehyde, dichloroacetyl chloride, CO, and phosgene with the new identified intermediates oxalyl chloride, trichloroacetyl chloride, and trichloroacetic acid are observed.운송 방법
UN1710 Trichloroethylene, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Purification Methods
Tricloroethylene undergoes decomposition in a similar way as CHCl3, giving HCl, CO, COCl2 and organic products. It reacts with KOH, NaOH and 90% H2SO4, and forms azeotropes with water, MeOH, EtOH, and acetic acid. It is purified by washing successively with 2M HCl, water and 2M K2CO3, then dried with K2CO3 and CaCl2, then fractionally distilled before use. It has also been steam distilled from 10% Ca(OH)2 slurry, most of the water being removed from the distillate by cooling to -30o to -50o and filtering off the ice through chamois skin: the trichloroethylene is then fractionally distilled at 250mm pressure and collected in a blackened container. [Carlisle & Levine Ind Eng Chem (Anal Ed) 24 1164 1932, Beilstein 1 IV 712.]비 호환성
Contact with strong caustics causes decomposition and the production of highly toxic and flammable dichloroacetylene. Violent reaction with chemically active metals; powders, or shavings, such as aluminum, barium, lithium, sodium, magnesium, and titanium. Violent reaction with aluminum in the presence of dilute hydrochloric acid. Thermal decomposition of trichloroethylene, due to contact with hot metal or UV radiation, forms hazardous products including chlorine gas, hydrogen chloride; and phosgene. Keep this chemical away from high temperatures, such as arc welding or cutting, unshielded resistance heating; open flames; and high intensity UV light. Slowly decomposed by light in presence of moisture, with formulation of hydrochloric acid.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. An alternative to disposal for TCE is recovery and recycling.트라이클로로에틸렌 준비 용품 및 원자재
원자재
LACQUER THINNER
펜타클로로에탄
염소(기체)
염화제이철무수물
탄화칼슘
사염화탄소
퍼클로로에틸렌
1,1,2,2-TETRACHLOROETHANE-D2
1,1,2,2-테트라클로로에틸렌
준비 용품
다이클로로아세틸 염화물
2-페닐페놀
디메토에이트
DIPROPETRYN
헥사메틸포스포릭트리아미드
Bis(2,3,3,3-tetrachloropropyl) ether
Pentoxyverine
(Z)-2-클로로-1-(2,4,5-트리클로로페닐)비닐 디메틸 인산염
chlorophyllin copper complex sodium salt
P-티몰
클로로아세트산
디클로르보스
시마진
3-AMINO-4,5,6,7-TETRAHYDRO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID METHYL ESTER
TETRACHLOROCYCLOPROPENE
펜타클로로에탄
Sulfadimethoxine sodium salt
헥사클로로에탄
4-Amino-6-methoxypyrimidine
2,4,6-TRIFLUOROPHENYL ISOTHIOCYANATE
캡산틴/캡소루빈
Chlorophyll A
2,2''-티오비스(4,6-디클로로페놀)
퍼클로로에틸렌
에틸렌티오우레아
p-페닐페놀
설파모노메타진
1,1,1-트라이클로로-2,2,2-트라이플루오로에테인
Cleaning agent
오르토-페닐펜산 나트륨
트라이클로로에틸렌 공급 업체
글로벌( 471)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Duling International Trade Co. LTD | +8618032673083 |
sales05@hbduling.cn | China | 15745 | 58 |
| BLiT (Hefei)Chemical Co.,Ltd | +86-551-62622640 |
sales@blitchem.com | China | 291 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7786 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 |
info@longyupharma.com | China | 2531 | 58 |