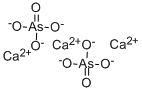비산 칼슘 C화학적 특성, 용도, 생산
개요
The calcium arsenate that is sold commercially today
as an insecticide is not a single chemical compound but
a complex mixture of several calcium arsenates and an
excess of calcium hydroxide. The material is made
from arsenic trioxide by first oxidizing it to arsenic pentoxide
with nitric acid and then reacting the solution of
arsenic pentoxide or arsenic acid with a slurry of
calcium hydroxide. The conditions of temperature,concentration, and duration of reaction are important
because of their influence on the physical nature of the
product.
화학적 성질
White powder. Slightly soluble in water;
soluble in dilute acids. Decomposes on heating.
물리적 성질
Commercial calcium arsenate generally is colored
pink and is alkaline in reaction. It is a finely divided
powder.
용도
Calcium arsenate has been used extensively against certain
insects affecting field crops, especially cotton. It cannot
be used safely on apples, peaches, beans, and some other
crops because of its burning effect on the foliage and
fruit. It is banned by a number of countries.
제조 방법
An old method involving electrolysis in solution for
the manufacture of calcium arsenate is as follows.
A solution of arsenious oxide in caustic soda
(As
2O
3:NaOH=198:250) is electrolyzed between iron
electrodes. Hydrogen and a small quantity of metallic
arsenic are liberated at the cathode and very little
oxygen at the anode, the basic arsenite in solution being
oxidized to arsenate. When this oxidation is complete,
any arsenic is filtered off and the solution treated with
milk of lime. A basic arsenate of extremely low solubility
is precipitated and after removal, is washed and dried.
일반 설명
White powder. Slightly soluble in water. Toxic by inhalation and ingestion. Used as an insecticide and germicide.
공기와 물의 반응
Slightly soluble in water.
반응 프로필
Has only weak oxidizing power but redox reactions can still occur. Soluble in dilute acids. [Hawley]. Produces toxic fumes of arsenic when heated to decomposition .
위험도
Toxic by ingestion and inhalation.
건강위험
CALCIUM ARSENATE is extremely toxic; the probable oral lethal dose for humans is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 150 lb. person. It is an irritant to eyes, respiratory tract, mouth and stomach. Damage to kidneys, liver and the nervous system have been reported. (Non-Specific -- Arsenic) Chronic exposure can cause bone marrow damage, often leading to aplastic anemia. There is epidemiological evidence that chronic ingestion of arsenic compounds causes a predisposition to skin cancers.
화재위험
Fire may produce irritating or poisonous gases. When heated to decomposition, CALCIUM ARSENATE produces toxic fumes of arsenic. Avoid heat. Hazardous polymerization may not occur.
Safety Profile
Confirmed human
carcinogen. Poison by ingestion. Moderately
toxic by skin contact. When heated to
decomposition it emits toxic fumes of
arsenic.
비산 칼슘 준비 용품 및 원자재
원자재
준비 용품