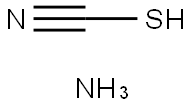
싸이오사이안산암모늄(로단암몬)
|
|
싸이오사이안산암모늄(로단암몬) 속성
- 녹는점
- 152-154 °C (lit.)
- 끓는 점
- 262.85°C
- 밀도
- 1.3
- 증기압
- <1 hPa (20 °C)
- 굴절률
- 1.5300 (estimate)
- 인화점
- 190 °C
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Specific Gravity
- 1.305
- 색상
- 투명 무색 또는 흰색
- 수소이온지수(pH)
- 4.8-5.8 (50g/l, H2O, 20℃)
- 냄새
- 냄새 없는
- pH 범위
- 4.5 - 6.0
- 수용성
- 163g/100mL(20℃)
- 감도
- Light Sensitive & Hygroscopic
- Merck
- 14,561
- BRN
- 3595135
- 노출 한도
- NIOSH: IDLH 25 mg/m3
- 안정성
- 안정적인. 강산, 강산화제와 호환되지 않습니다. 질산납과 폭발성 혼합물을 형성합니다.
- LogP
- 0.58
- CAS 데이터베이스
- 1762-95-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/21/22-32-52/53-36/37/38 | ||
| 안전지침서 | 13-61-36/37-36-26-46 | ||
| 유엔번호(UN No.) | 9092 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | XK7875000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| HS 번호 | 28380000 | ||
| 유해 물질 데이터 | 1762-95-4(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 500 mg/kg | ||
| 기존화학 물질 | KE-01750 |
싸이오사이안산암모늄(로단암몬) C화학적 특성, 용도, 생산
화학적 성질
Ammonium thiocyanate is a colorless solid which absorbs moisture, becoming liquid, known as the “liquor.”용도
Colorless deliquescent crystals made by boiling an aqueous solution of ammonium cyanide with sulfur or polysulfides. It must be kept in a well-sealed container. The principal use for ammonium thiocyanate was in gold-toning formulas, but it was also used in a 5 percent solution to dissolve gelatin in overexposed carbon prints.생산 방법
Ammonium thiocyanate (plus ammonium sulfide) [CAS: 12135-76-1] may be made by reaction of ammonia and carbon disulfide, a reaction which probably accounts for the presence of ammonium thiocyanate in the products of the destructive distillation of coal. This reaction corresponds to the formation of ammonium cyanate from ammonia and carbon dioxide.제조 방법
(NH4)2S is obtained from reacting hydrogen sulfide with excess of ammonia:H2S + 2NH3 → (NH4)2S.
정의
ammonium thiocyanate: A colourless, soluble crystalline compound,NH4NCS. It is made by theaction of hydrogen cyanide on ammoniumsulphide or from ammoniaand carbon disulphide in ethanol. Onheating, it turns into its isomerthiourea, SC(NH2)2. Its solutions givea characteristic blood-red colour withiron(III) compounds and so are employedas a test for ferric iron. Ammoniumthiocyanate is used as arapid fixative in photography and asan ingredient in making explosives.일반 설명
Ammonium thiocyanate is a colorless crystalline solid. Ammonium thiocyanate is soluble in water. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium thiocyanate is used in chemical analysis, in photography, as a fertilizer, and for many other uses.공기와 물의 반응
Water soluble.반응 프로필
Ammonium thiocyanate can release ammonia vapors if mixed with a chemical base or with an acid. Violent or explosive reactions have occurred when thiocyanates are mixed with oxidizing agents (such as chlorates(potassium chlorate), nitrates, nitric acid, and peroxides). Nitric acid violently oxidized a thiocyanate solution [Bretherick 1979 p. 121]. An explosion of guanidine nitrate demolished an autoclave built to withstand 50 atmospheres, in which Ammonium thiocyanate was being made from Ammonium thiocyanate and lead nitrate [C. Angew. Chem. 49:23 1936].건강위험
Inhalation of dust causes irritation of nose and throat. Ingestion causes dizziness, cramps, nervous disturbances. Dust irritates eyes. Can be absorbed through skin; prolonged contact may produce various skin eruptions, dizziness, cramps, nausea, and mild to severe disturbance of the nervous system.화재위험
Special Hazards of Combustion Products: Decomposes to form ammonia, hydrogen sulfide, and hydrogen cyanide. Oxides of nitrogen may also form. All of these products are toxic.Safety Profile
Poison by ingestion and intraperitoneal routes. Human systemic effects by ingestion: hallucinations and distorted perceptions, nausea or vomiting, and other gastrointestinal effects. See also THIOCYANATES. When heated to decomposition it emits toxic fumes of NH3, NOx, SO,, and CN-. Incompatible with KClO3 and mixtures with Pb(NO3)2.잠재적 노출
It has many uses in making matches, fabric processing; metals processing; chemical manufacturing, electroplating; zinc coating; liquid rocket propellents, fabric dyeing, polymerization catalyst, in photography. Used as a laboratory chemical. May be used as an agricultural chemical: herbicides, weed killers, defoliants.운송 방법
UN2672 Ammonia solutions, relative density between 0.880 and 0.957 at 15 C in water, with . 10 % but not . 35 % ammonia, Hazard class: 8; Labels: 8- Corrosive material. UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9- Miscellaneous hazardous material, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Purification Methods
Crystallise it three times from dilute HClO4 to give material optically transparent at wavelengths longer than 270nm. It has also been crystallised from absolute MeOH or from acetonitrile.비 호환성
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Acts as an acid; incompatible with lead nitrate, chlorates, nitric acid, acid, acid fumes. In the presence of moisture, this chemical is corrosive to brass, copper, iron. Ammonium thiocyanate liquor can release ammonia vapors if mixed with a chemical base or with an acid. Violent or explosive reactions have occurred when thiocyanates are mixed with oxidizing agents . Nitric acid violently oxidized a thiocyanate solution . An explosion of guanidine nitrate demolished an autoclave built to withstand 50 atmospheres, in which it was being made from ammonium thiocyanate and lead nitrate .폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Slowly add to large container of water. Stir in slight excess of soda ash. Decant or siphon liquid from sludge, neutralize with HCl and flush to sewer. Sludge may be landfilled.싸이오사이안산암모늄(로단암몬) 준비 용품 및 원자재
원자재
준비 용품
티오시안산, 수은
티오세미카바지트
[5-(프로필시오)-1H-벤지마이다졸-2-일]카르바믹산 메틸 에스테르; 알벤다졸
2,3,5-TRICHLOROBENZENEBORONIC ACID
Acetazolamide
5-아미노-1,3,4-티아디아졸-2-티올
티오시안산칼슘
1-PHENYL-3-THIOSEMICARBAZIDE
4-(DIMETHYLAMINO)PHENYL THIOCYANATE
2-AMINO-5,6-DIMETHYLBENZOTHIAZOLE
싸이오사이안산 나트륨
1-(2-Chlorophenyl)-2-thiourea
Benazolin
Rhodanine-3-acetic acid
브푸로페진
카보닐 황화합물
포스티에탄
2-(ACETAMIDO)-5-(CHLOROSULFONYL)-1,3,4-THIADIAZOLE
OMEPRAZOLE
N-(2-Methylphenyl)thiourea
1-나프틸싸이오요소(안투)
2,5-디시오 비우레아
4,5-DIPHENYL-2-IMIDAZOLETHIOL
4-(Propylthio)benzene-1,2-diamine
트리시크라졸
8-BROMO-4-CHLORO-2-METHYLTHIOPYRAZOLO[1,5-A]1,3,5-TRIAZINE
Moxonidine
티오황산 암모늄
2-AMINO-6-HYDROXY-PYRIMIDINE-4-CARBOXYLIC ACID
싸이오사이안산 칼륨
알릴 아이소사이오사이아네이트
BISMERTHIAZOL
Tert-뷰틸 아이소티오사이안산
4-BROMOPHENYLTHIOUREA
티오요소
싸이오사이안산암모늄(로단암몬) 공급 업체
글로벌( 656)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 |
sales01@sdlookchemical.com | China | 2739 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12457 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 |
daisy@crovellbio.com | China | 5964 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 |
admin@hbdangtong.com | China | 991 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7613 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 |
admin@juchuangchem.com | China | 387 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Nanjing Finetech Chemical Co., Ltd. | 025-85710122 17714198479 |
sales@fine-chemtech.com | CHINA | 885 | 55 |