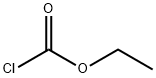
클로로포름산에틸
|
|
클로로포름산에틸 속성
- 녹는점
- -81 °C (lit.)
- 끓는 점
- 93 °C (lit.)
- 밀도
- 1.135 g/mL at 25 °C (lit.)
- 증기 밀도
- 3.74 (vs air)
- 증기압
- 3.42 psi ( 20 °C)
- 굴절률
- n
20/D 1.395(lit.)
- 인화점
- 57 °F
- 저장 조건
- 2-8°C
- 용해도
- 클로로포름(수용성), 에틸아세테이트(약간 용해됨)
- 물리적 상태
- 액체
- 색상
- 흰색에서 황백색까지
- 냄새
- 화나게 하는; 염산처럼 날카롭다.
- 수용성
- 물에서 분해되다
- 감도
- Moisture Sensitive
- Merck
- 14,3784
- BRN
- 385653
- Dielectric constant
- 11.3(20℃)
- 안정성
- 수분에 민감한
- LogP
- 1.440 (est)
- CAS 데이터베이스
- 541-41-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,T+,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-22-26-34-50 | ||
| 안전지침서 | 9-16-26-28-33-36/37/39-45-61 | ||
| 유엔번호(UN No.) | UN 1182 6.1/PG 1 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | LQ6125000 | ||
| F 고인화성물질 | 10-19-21 | ||
| 자연 발화 온도 | 842 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | I | ||
| HS 번호 | 29151300 | ||
| 유해 물질 데이터 | 541-41-3(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-05692 |
클로로포름산에틸 C화학적 특성, 용도, 생산
화학적 성질
Ethyl chloroformate is a colorless to light yellow liquid that is corrosive and flammable. It is prepared from phosgene and ethanol. It has a sharp pungent odor, like hydrochloric acid, and it decomposes in water. It is miscible with alcohol, benzene, chloroform, and ether.용도
Ethyl chloroformate (chloroformic acid ethyl ester) is used as a solvent in the photographic industry, and as a chemical intermediate in the production of various carbamates, and used in synthesis of dyes, drugs, veterinary medicines, herbicides, and insecticides. It is also used in the production of flotation agents for ores, as a stabilizer for PVC, and in the production of modified penicillins and heterocyclic compounds (Gerhartz, 1985).Cathyl Chloride is used in the preparation of new inhibitors for β-homocysteine S-methyltransferase. Also used in the synthesis of a hexosaminidase inhibitor.
일반 설명
A colorless liquid with a pungent odor. Flash point 66°F. Very toxic by inhalation. Corrosive to metals and tissue. Vapors are heavier than air. Prolonged exposure to low concentrations or short exposure to high concentrations may have adverse health effects from inhalation.공기와 물의 반응
Highly flammable. Emits fumes containing HCl on contact with moist air. Decomposes exothermically but slowly in water.반응 프로필
Ethyl chloroformate decomposes slowly in water to form ethanol, HCl, and CO2 Attacks many metals especially in humid atmosphere [Handling Chemicals Safely 1980. p. 476]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].건강위험
Inhalation causes mucous membrane irritation, coughing, and sneezing. Vapor causes severe lachrymation; liquid causes acid-type burns of eyes and skin, like those of hydrochloric acid. Ingestion causes severe burns of mouth and stomach.화재위험
Special Hazards of Combustion Products: Toxic chlorine and phosgene gases may be formed in fires.화학 반응
Reactivity with Water: Slow reaction with water, evolving hydrogen chloride (hydrochloric acid); Reactivity with Common Materials: Slow evolution of hydrogen chloride from surface moisture reaction can cause slow corrosion; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water, rinse with sodium bicarbonate or lime solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Safety Profile
Poison by ingestion, inhalation, and intraperitoneal routes. Moderately toxic by skin contact. Corrosive. An eye, skin, and mucous membrane irritant. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidzing materials. Reacts with water or steam to produce toxic and corrosive fumes. To fight fire, use CO2, dry chemical. When heated to decomposition it emits highly toxic fumes of Cl-.잠재적 노출
Heavily used in industry for various processes; in ore processing, photography, making other chemicals including amines, carbamates, isocyanates; polymers, diethyl carbonate; nitriles, etc.운송 방법
UN1182 Ethyl chloroformate, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, 8-Corrosive material Inhalation Hazard Zone BPurification Methods
Wash the ester several times with water, redistil it using an efficient fractionating column at atmospheric pressure and a CaCl2 guard tube to keep free from moisture [Hamilton & Sly J Am Chem Soc 47 435 1925, Saunders et al. J Am Chem Soc 73 3796 1951]. [Beilstein 3 IV 23.] LACHRYMATORY AND TOXIC.비 호환성
Highly flammable; Vapors may form explosive mixture with air. Emits fumes containing HCl on contact with moist air. Decomposes exothermically but slowly in water. Ethyl chloroformate decomposes slowly in water forming ethanol, hydrogen chloride and carbon dioxide. May react vigorously, possibly explosively, if mixed with di-isopropyl ether or other ethers in the presence of trace amounts of metal salts. Reacts with acids, alkalies, amines, alcohols, oxidizers and water. Corrosive to metals especially in the presence of moisture.폐기물 처리
Use a licensed professional waste disposal service to dispose of this material. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices클로로포름산에틸 준비 용품 및 원자재
원자재
준비 용품
6-Fluoro-4-(trifluoromethyl)quinolin-2-amine ,97%
ETHYL 2-[(2-THIENYLCARBONYL)AMINO]ACETATE
ETHYL 5-CHLOROTHIOPHENE-2-CARBOXYLATE
ETHYL 1-METHYL-1H-IMIDAZOLE-2-CARBOXYLATE
5-Bromopicolinic acid
ethyl N-cyano-N-methylaminoformate
Molsidomine
DELAPRIL
N-(ETHOXYCARBONYL)-N-(ETHOXYCARBONYKLETHYL)GLYCINE ETHYL ESTER
Gabapentin
NEMONAPRIDE
Carbimazole
4-(Trifluoromethyl)quinoline-2-carbohydrazide ,97%
(6-Fluoro-4-(trifluoromethyl)quinolin-2-yl)methanol ,97%
Ethyl 6-fluoro-4-(trifluoromethyl)quinoline-2-carboxylate ,97%
3-Thienylmethanol
ethyl 1-methylpyrrole-2-carboxylate
ethyl 1,4-diazepane-1-carboxylate
Loratadine
3-Chlorothiophene-2-carboxylic acid
아목사핀
5-CHLORO-2-THIOPHENECARBOXYLIC ACID HYDRAZIDE
Ethyl 1-methyl-1H-pyrazole-5-carboxylate
N-(Ethoxycarbonyl)-4-ethoxycarbonyl-1-methylpyrazole-5-sulfonamide
ETHYL 3-(6-METHOXY-2-NAPHTHYL)-3-METHYL GLYCIDATE
FLUMICLORAC-PENTYL
(4-(Trifluoromethyl)quinolin-2-yl)methanol ,97%
로테논
Flupirtine
DIETHYL HYDRAZODICARBOXYLATE
ETHYL (3-HYDROXYPHENYL)CARBAMATE
Bonnecor
Methyl 1-Methyl-2-oxopyrrolidine-3-carboxylate
5-CHLORO-2-FLUOROPHENYL ISOTHIOCYANATE
5-머캅토-1-메틸테트라졸
Ethyl thiophene-3-carboxylate
CINITAPRIDE HYDROGEN TARTRATE
N-METHYLURETHANE
3-CHLORO-4-FLUOROPHENYL ISOTHIOCYANATE
4-TRIFLUOROMETHYL-QUINOLINE-2-CARBOXYLIC ACID