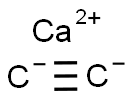탄화칼슘
|
|
탄화칼슘 속성
- 녹는점
- 447°C
- 끓는 점
- 2300°C
- 밀도
- 2.22 g/mL at 25 °C(lit.)
- 저장 조건
- water-free area
- 용해도
- reacts with H2O
- 물리적 상태
- 플레이크 크리스탈
- 색상
- 회색-검정색
- Specific Gravity
- 2.22
- 수용성
- 가수분해
- 감도
- Moisture Sensitive
- Merck
- 14,1656
- BRN
- 3909011
- Dielectric constant
- 5.8 - 7.0(0.0℃)
- 노출 한도
- ACGIH: TWA 2 mg/m3
OSHA: TWA 5 mg/m3
NIOSH: IDLH 25 mg/m3; TWA 2 mg/m3
- 안정성
- 안정성 수분을 방출하는 인화성 가스(아세틸렌)와 격렬하게 반응합니다. 이 물질에 화재가 발생한 경우 물을 사용하지 마십시오. 습기, 물, 강산화제, 알코올, 염화수소, 마그네슘과 혼합되지 않습니다.
- InChIKey
- UIXRSLJINYRGFQ-UHFFFAOYSA-N
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 15-41-37/38 | ||
| 안전지침서 | 8-43-43A-39-26 | ||
| 유엔번호(UN No.) | UN 1402 4.3/PG 2 | ||
| WGK 독일 | 1 | ||
| F 고인화성물질 | 10-21 | ||
| TSCA | Yes | ||
| 위험 등급 | 4.3 | ||
| 포장분류 | II | ||
| HS 번호 | 28491000 | ||
| 유해 물질 데이터 | 75-20-7(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-04470 |
탄화칼슘 C화학적 특성, 용도, 생산
개요
Calcium carbide,is a binary salt. It is a grayish-black hard solid that reacts with water to produce acetylene gas, a solid corrosive that is calcium hydroxide, and release heat. Acetylene gas is manufactured by reacting calcium carbide with water. Because acetylene is so unstable, it is not shipped in bulk quantities.Calcium carbide is shipped to acetylene-generating plants where it is reacted with water in a controlled reaction. After the reaction process, the acetylene gas is placed into specially designed containers with a honeycombed mesh inside for shipment and use. It is dissolved in acetone for stability. Calcium carbide has a specific gravity of 2.22, which is heavier than water. The four-digit UN identification number for calcium carbide is 1402. The NFPA 704 designation is health 3, flammability 3, and reactivity 2. The white section at the bottom of the diamond contains a W with a slash through it, indicating water reactivity. It is shipped in metal cans, drums, and specially designed covered bins on railcars and trucks. When shipped and stored, it should be kept in a cool, dry place. Primary uses are in the generation of acetylene gas for welding, vinyl acetate monomer, and as a reducing agent.
화학적 성질
grey or black solid with a garlic-like odour물리적 성질
Grayish-black orthorhombic crystal; density 2.22 g/cm3; melts at 2,200°C; reacts with water.용도
Calcium carbide is the most relevant carbide industrially because of its important role as the basis of acetylene industry. In locations where there is shortage of petroleum, Calcium Carbide is used as the starting material for the production of acetylene (1 kg of carbide yields ~300 liters acetylene), which, in turn, can be used as a building block for a range of organic chemicals (e.g. vinyl acetate, acetaldehyde and acetic acid). In some locations, acetylene is also used to produce vinyl chloride, the raw material for the production of PVC.A less important use of Calcium Carbide is related to the ferilizers industry. It reacts with nitrogen to form calcium cyanamide, which is the starting material for the production of cyanamide (CH2N2). Cyanamide is a common agricultural product used to stimulate early foliation.
Calcium Carbide can also be employed as desulfurizing agent for producing low-sulfur carbon steel. Also, it is used as a reducing agent to produce metals from their salts, e.g., for direct reduction of copper sulfide to metallic copper.
제조 방법
Calcium carbide (CaC2) is manufactured by heating a lime and carbon mixture to 2000 to 2100°C (3632 to 3812°F) in an electric arc furnace. At those temperatures, the lime is reduced by carbon to calcium carbide and carbon monoxide (CO), according to the following reaction: CaO + 3C → CaC2 + COLime for the reaction is usually made by calcining limestone in a kiln at the plant site. The sources of carbon for the reaction are petroleum coke, metallurgical coke, and anthracite coal. Because impurities in the furnace charge remain in the calcium carbide product, the lime should contain no more than 0.5 percent each of magnesium oxide, aluminum oxide, and iron oxide, and 0.004 percent phosphorus. Also, the coke charge should be low in ash and sulfur. Analyses indicate that 0.2 to 1.0 percent ash and 5 to 6 percent sulfur are typical in petroleum coke. About 991 kilograms (kg) (2,185 pounds [lb]) of lime, 683 kg (1,506 lb) of coke, and 17 to 20 kg (37 to 44 lb) of electrode paste are required to produce 1 megagram (Mg) (2,205 lb) of calcium carbide.
화학 반응
Calcium carbide is grayish-black solid, reacts with water yielding acetylene gas and calcium hydroxide, formed at electric furnace temperature from calcium oxide and carbon.일반 설명
Grayish-black irregular lump solid. Used to make acetylene and in steel manufacture.공기와 물의 반응
Reacts rapidly with water to generate the flammable gas acetylene and the base calcium hydroxide. Enough heat may be generated to ignite the gas [Jones, G.W. BM Report Invest. 3755 1944].반응 프로필
Calcium carbide is a reducing agent. May react vigorously with oxidizing materials. The powdered mixture of the acetylide and iron oxide and iron chloride burns violently upon ignition, producing molten iron. Calcium carbide incandesces with chlorine, bromine, or iodine at 245, 350, or 305°C., respectively, [Mellor, 1946, Vol. 5, 862]. The carbide burns incandescently when mixed and heated with lead difluoride, magnesium, hydrogen chloride, and tin (II) chloride, [Mellor, 1946, 1940, 1946, and 1941], respectively. Interaction of Calcium carbide with methanol to give calcium methoxide is vigorous , but subject to an induction period of variable length. Once reaction starts, evolution of acetylene gas is very rapid, unpublished observations [Bretherick 1995]. Mixing Calcium carbide with silver nitrate solutions forms silver acetylide, a highly sensitive explosive. Copper salt solutions would behave similarly, [Photogr. Sci. Eng., 1966, 10, 334]. The mixture of Calcium carbide and sodium peroxide is explosive, as is Calcium carbide and perchloryl fluoride as gases at 100-300°C.위험도
Forms flammable and explosive gas and corrosive solid with moisture.건강위험
It is a corrosive solid. Because it is highlywater-reactive, skin contact can cause burn.화재위험
Behavior in Fire: If wet by water, highly flammable acetylene gas is formed.Safety Profile
Reaction on contact with moisture forms explosive acetylene gas. Flammable on contact with moisture, acid or acid fumes; evolves heat or flammable vapors. Moderate explosion hazard. Incandescent reaction with Cl2 (245℃), Brz (350℃), IS (305℃), HCl gas + heat, PbF2, Mg + heat. Incompatible with Se, (KOH + Ch), AgNO3, Na2O2, SnCl2, S, water. Mixtures with iron(IⅡ) chloride, iron(IⅡ) oxide, tin(Ⅱ) chloride are easily ignited and burn fiercely. Vigorous reaction with methanol after an induction period. Addttion to silver nitrate solutions precipitates the dangerously explosive silver acetylide. Copper salt solutions behave similarly. See also CALCIUM HYDROXIDE and ACETYLENE.탄화칼슘 준비 용품 및 원자재
원자재
준비 용품
탄화칼슘 공급 업체
글로벌( 160)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12453 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 |
info@longyupharma.com | China | 2531 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; |
laboratory@coreychem.com | China | 30255 | 58 |