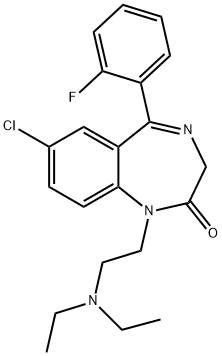
FLURAZEPAM
|
|
FLURAZEPAM 속성
- 녹는점
- 77-82°
- 끓는 점
- 551.4±50.0 °C(Predicted)
- 밀도
- 1.2089 (estimate)
- 인화점
- 9℃
- 저장 조건
- 2-8°C
- 용해도
- 45%(w/v) 수성 2-히드록시프로필-β-시클로덱스트린: <6mg/mL
- 산도 계수 (pKa)
- pKa 1.90±0.05(H2O) (Uncertain);8.16±0.05 (Uncertain)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 연노랑
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,T,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-33-39/23/24/25-23/24/25-11 | ||
| 안전지침서 | 1/2-22-36-45-36/37-16-7 | ||
| 유엔번호(UN No.) | 3249 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | DF0875000 | ||
| 위험 등급 | 6.1(b) | ||
| 포장분류 | III | ||
| 유해 물질 데이터 | 17617-23-1(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-05592 |
FLURAZEPAM C화학적 특성, 용도, 생산
개요
Flurazepam (CRM) (Item No. 18160) is a certified reference material that is structurally categorized as a benzodiazepine. It acts as a partial agonist of the benzodiazepine site on GABAA receptors, potentiating the action of GABA with an EC50 value of 930 nM in neuronal chick spinal cord cultures. Through this signaling mechanism, flurazepam produces anxiolytic and sedative properties that have been implemented clinically in the treatment of insomnia but also carry a potential for misuse. This product is intended for forensic and research purposes.화학적 성질
Flurazepam is a pale yellow crystalline solid.용도
Anticonvulsant; relaxant (muscle); sedative-hypnotic Dalmane (Valeant.Pharmacokinetics
Chlorazepate is yet another benzodiazepine that is rapidly metabolized (3-decarboxylation) to N-desmethyldiazepam and so shares similar clinical and pharmacokinetic properties to chlordiazepoxide and diazepam.부작용
The half-life of flurazepam is fairly long (~7 hours); consequently, it has the same potential as chlordiazepoxide and diazepam to produce cumulative clinical effects and side effects (e.g., excessive sedation) and residual pharmacological activity, even after discontinuation.Safety Profile
Poison by intravenous routes. Moderately toxic by ingestion and subcutaneous routes. Experimental reproductive effects. Caution: May be habit forming. This is a controlled substance (depressant) listed in the US. Code of Federal Regulations, Title 21 Part 1308.14. When heated to decomposition it emits very toxic fumes of Cl-, Fand NOx. See also DIAZEPAM.잠재적 노출
A Drug. Flurazepam is used as a seda tive in capsules or liquid form.신진 대사
Flurazepam is administered orally as the dihydrochloride salt. It is rapidly 1N-dealkylated to give the 2′-fluoro derivative of N-desmethyldiazepam, and it subsequently follows the same metabolic pathways as chlordiazepoxide and diazepam.운송 방법
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.폐기물 처리
It is inappropriate and possi bly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quanti ties of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.FLURAZEPAM 준비 용품 및 원자재
원자재
플로로(2-)벤조일
(3S)-3-(tert-Butoxycarbonyl)amino-1-chloro-4-phenyl-2-butanone
염화에틸
클로로아세틸 클로라이드
염화 암모늄
P-클로로아닐린
Aza
헥사민
디에틸 카보네이트
이염화에탄
메틸산 나트륨
(2-CHLORO-ETHYL)-DIETHYL-AMINE
준비 용품
FLURAZEPAM 관련 검색:
메토트렉세이트 다이아제팜
Fludiazepam
1,2-ETHANEDIAMINE, N-ETHYL-N',N'-DIMETHYL-N-PHENYL-
Nordazepam
Benzophenone imine
1,3-Dihydro-5-phenyl-1,4-benzodiazepin-2-one
N-desalkylflurazepam
FLURAZEPAM
MEDAZEPAM
FLURAZEPAM DIHYDROCHLORIDE
4-CHLORO-2-METHYLACETANILIDE
NETROPSIN DIHYDROCHLORIDE HYDRATE
3-PIPERIDIN-3-YLMETHYLPYRIDINE DIHYDROCHLORIDE
FLURAZEPAM [CONTROLLED SUBSTANCE] N
3-PIPERIDIN-4-YLMETHYLPYRIDINE DIHYDROCHLORIDE
4-PIPERIDIN-4-YLMETHYLPYRIDINE DIHYDROCHLORIDE
3-PYRROLIDIN-2-YLMETHYLPYRIDINE DIHYDROCHLORIDE