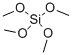
테트라메톡시실란 C화학적 특성, 용도, 생산
용도
이 제품은 주로 전자 산업, 광학 유리 및 실리콘 합성을위한 처리제 및 응고제의 절연재로 사용됩니다.
그것은 내열성, 내 화학성 코팅, 실리콘 솔벤트 및 정밀 주조 접착제를 생산하는 데 사용됩니다. 그것은 유기 합성에서 널리 사용되는 중간체입니다.
그것은 또한 분말 가공에 사용됩니다.
화학적 성질
Tetramethyl orthosilicate (TMOS), the methyl ester of orthosilicic acid, is a colorless, low-viscosity liquid. It is industrially the most important of the tetraalkyl silicates.
용도
Tetramethyl Orthosilicate is a compound used in the research of the multifunctionality of silicified nanoshells and the efficiency at adsorbing cadmium ions at cell interfaces.
생산 방법
Silica aerogels are usually prepared by base-catalyzed reaction
of tetramethoxysilane or tetraethoxysilane, mostly with
ammonia as the catalyst. A modification of this procedure is
to prehydrolyze Si(OR)4 with a small amount of water under
acidic conditions.
일반 설명
A clear colorless liquid. Flash point below 125°F. Less dense than water and insoluble in water. Very toxic by ingestion and inhalation and very irritating to skin and eyes. Used to make paints and lacquers.
공기와 물의 반응
Flammable. Insoluble in water.
반응 프로필
Tetramethyl orthosilicate is incompatible with the following: Oxidizers; hexafluorides of rhenium, molybdenum & tungsten .
위험도
Eye damage and upper respiratory tract irri-tant.
건강위험
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Bromoacetates and chloroacetates are extremely irritating/lachrymators. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
화재위험
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Poison by
intraperitoneal route. Moderately toxic by
inhalation. Midly toxic by skin contact. A
severe eye irritant. This material can cause extensive necrosis (experimentally),
keratoconus, and opaque cornea. It also
causes severe human eye injuries, as well as
necrosis of corneal cells, which progresses
long after exposure has ceased. It is
destructive and its effects resist treatment.
Permanent blindness is possible from
exposure to it. The kidney seems to be most
subject to injury regardless of the mode of
exposure. Pulmonary edema has also
occurred. This material is more toxic than
either ethyl silicate or silicic acid, although it
has been thought that the injury caused is
largely due to the action of the silicic acid.
Flammable when exposed to heat or flame;
can react vigorously with oxidizing
materials. Potentially violent reaction with
metal hexafluorides (e.g., rhenium,
molybdenum, tungsten). When heated to
decomposition it emits acrid smoke and
irritating fumes.
잠재적 노출
Methyl silicate is used in coating
screens of television picture tubes. It may be used in mold
binders and in corrosion-resistant coatings; as well as in
catalyst preparation and as a silicone intermediate.
운송 방법
UN2606 Methyl orthosilicate, Hazard class: 6.1;
Labels: 6.1-Poisonous materials, 3-Flammable liquid.
Purification Methods
Purification is as for tetraethoxysilane. It has a vapour pressure of 2.5mm at 0o. [IR: Sternbach & MacDiarmid J Am Chem Soc 81 5109 1959. Beilstein 1 IV 1266.]
비 호환성
Vapor may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, including alkaline earth
metals, metals, strong acids, strong bases; water, moisture,
steam decomposes releasing toxic, flammable gases.
Violent reaction with metal hexafluorides of rhenium,
molybdenum, and tungsten. Contact with metals may
evolve flammable hydrogen gas.
테트라메톡시실란 준비 용품 및 원자재
원자재
준비 용품