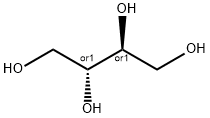
에리트리톨
|
|
에리트리톨 속성
- 녹는점
- 118-120 °C (lit.)
- 끓는 점
- 329-331 °C (lit.)
- 밀도
- 1,451 g/cm3
- 굴절률
- 1.4502 (estimate)
- FEMA
- 4819 | ?ERYTHRITOL
- 인화점
- 329-331°C
- 저장 조건
- -20°C
- 용해도
- H2O: 0.1 g/mL, 투명 내지 거의 투명, 무색
- 물리적 상태
- 결정질 분말 또는 결정
- 산도 계수 (pKa)
- 13.9(at 25℃)
- 색상
- 흰색에서 황백색까지
- 냄새
- 100.00%. 냄새 없는
- 수용성
- 녹는
- Merck
- 14,3675
- BRN
- 1719753
- 안정성
- 안정적인. 강한 산화제와 호환되지 않습니다.
- InChIKey
- UNXHWFMMPAWVPI-ZXZARUISSA-N
- LogP
- -2.996 (est)
- CAS 데이터베이스
- 149-32-6(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | KF2000000 | ||
| F 고인화성물질 | 3-10 | ||
| TSCA | Yes | ||
| HS 번호 | 29054910 | ||
| 유해 물질 데이터 | 149-32-6(Hazardous Substances Data) | ||
| 독성 | LD50 in male, female rats (g/kg): 6.6, 9.6 i.v.; >16, >16 s.c.; 13.1, 13.5 orally (Munro) | ||
| 기존화학 물질 | KE-13121 |
에리트리톨 C화학적 특성, 용도, 생산
물성
성상 : 결정, 색상 : 백색, 녹는점/어는점 : 121.5 ℃, 초기 끓는점과 끓는점 범위 : 329~331 ℃, 증기압 : 0.0000237 ㎜Hg (25℃(EXP)), 용해도 : 610000 ㎎/ℓ (22℃(EXP)), 비중 : 1.451 (20℃), n-옥탄올/물분배계수 (Kow) : -2.29 (EXP)(Log Kow).개요
에리스리톨은 내열성이 뛰어나 200℃에서 1시간 동안 가열하여도 분해되지 않을 뿐만 아니라, 흡습성도 낮아서 결정을 쉽게 형성하는 장점을 가지고 있다.감미도는 설탕의 70∼80%의 감미를 가지고 있고 용해시에 흡열작용이 매우 강하여 청량감이 뛰어나다. 또한 장점 중에 대표적인 것은 저칼로리 식품이라는 것인데 다른 감미료와 달리 체내에서 에너지원으로 이용되지 않고 소변으로 대부분 배출된다. 그 밖에 충치균 등에 의해 이용되지 않기 때문에 충치를 일으키지도 않는다.
용도
식첨용 용도로는 향미증강제, 감미료, 습윤제 등에 사용된다.순도시험
(1) 환원당(글루코오스로서) : 이 품목 500mg을 정밀히 달아 물 2mL에 녹이고 흔들어준 다음 펠링시액 2mL를 가해주고 끓을 때까지 가열한 후 냉각시킨 액을 시험용액으로 하고, 따로 글루코오스용액(1mL당 0.75mg 글루코오스 함유) 2mL에 펠링시액 2mL를 가해주고 끓을 때까지 가열한 후 냉각시킨 액을 표준용액으로 하여 양 액을 비교할 때, 시험용액에 생성된 침전물은 표준용액에 생성된 적갈색 침전물보다 적어야 한다(0.3% 이하).
(2) 리비톨 및 글리세롤 : 이 품목을 정량법에 따라 시험하고 다음 계산식에 따라 리비톨 및 글리세롤의 함량을 구할 때, 그 합계는 0.1% 이하이어야 한다. 다만, 에리스리톨, 글리세롤 및 리비톨 각각에 대한 상대적유지시간은 1.0, 1.10 및 0.93이다.
(3) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 0.5ppm 이하이어야 한다.
확인시험
(1) 이 품목은 물에 잘 녹고, 에탄올에는 약간 녹으며 에테르에는 녹지 않는다.
(2) 이 품목의 융점은 119~123℃이어야 한다.
(3) 이 품목을 정량법에 따라 시험할 때, 시험용액의 주피크와 에리스리톨 표준용액의 피크의 유지시간이 일치한다.
정량법
이 품목을 건조한 다음 약 2g을 정밀히 달아 물을 가하여 50mL로 한 액을 시험용액으로 한다. 따로, 미리 건조시킨 에리스리톨표준품 2g을 정밀히 달아 50mL로 한 액을 표준용액으로 한다. 시험용액 및 표준용액 각각 10μL씩을 다음의 조작조건으로 액체크로마토그래피에 주입하고 다음 계산식에 따라 에리스리톨의 함량을 구한다.
조작조건
검 출 기 : 시차굴절계(RI detector)
칼 럼 : MCI-CKO8SH, Shodex KC811 또는 이와 동등한 것
칼럼온도 : 60℃
이 동 상 : 물
유 속 : 1.0mL/min
정의
이 품목은 효모인 Moniliella pollinis, Trichosporonoides megachilensis 또는 Candida lipolytica(Yarrowia lipolytica)에서 얻어진 발효액을 여과, 정제, 결정화, 수세를 거친 다음 건조하여 얻어지는 물질로서 그 성분은 에리스리톨이다.
강열잔류물
이 품목 2g을 취하여 강열잔류물시험법에 따라 시험할 때, 그 양은 0.1% 이하이어야 한다.
화학적 성질
Erythritol (meso-erythritol, meso-1,2,3,4-Tetrahydroxybutan) has been known for a long time. Its potential use as a bulk sweetener was, however, recognized rather late.Erythritol is a natural constituent of several foods and beverages in levels sometimes exceeding 1 g/kg. Its solubility in water is approximately 370 g/L at room temperature and increases with increasing temperature. Erythritol melts at 121 C and is stable up to more than 160 C and in a pH range from 2 to 10. Depending on the concentration used, erythritol is approximately 60 % as sweet as sucrose. It is noncariogenic and not metabolized in the human body which means that it is more or less calorie-free.
In the European Union, erythritol is approved as E 968 for a large number of food applications. It is GRAS in the United States and also approved in many other countries.
용도
Erythritol is a sweetener (polyol) manufactured by fermentation of glucose, the glucose-rich substrate being obtained by the enzymatic hydrolysis of starch. it is 60–70% as sweet as sugar, has excellent heat and acid stability, a high digestive tolerance, and a caloric value of 0.2 kcal/g. it is the only polyol produced by fermentation. it can be used as a sugar replacement in confectioneries, beverages, and desserts.정의
ChEBI: The meso-diastereomer of butane-1,2,3,4-tetrol.생산 방법
Erythritol is a starch-derived product. The starch is enzymatically hydrolyzed into glucose which is turned into erythritol via a fermentation process, using osmophilic yeasts or fungi (e.g. Moniliella pollinis, or Trichosporonoides megachiliensis).일반 설명
meso-Erythritol, belonging to the class of sugar alcohols, is identified in a variety of food products, fruits, vegetables, beverages and dietary supplements. It is known as a low glycemic food additive and plays an important role as a sweetener for diabetic patients, since it does not have glycemic or insulinemic effect due to its ability to not get metabolized but get absorbed in the small intestine. It is also reportedly used as a sugar substitute in toothpaste, chewing gums, confectionery food products, etc.Pharmaceutical Applications
Erythritol is a naturally occurring noncariogenic excipient used in a variety of pharmaceutical preparations, including in solid dosage forms as a tablet filler, and in coatings. It has also been investigated for use in dry powder inhalers.It is also used in sugar-free lozenges,and medicated chewing gum.Erythritol can also be used as a diluent in wet granulation in combination with moisture-sensitive drugs. In buccal applications, such as medicated chewing gums, it is used because of its high negative heat of solution which provides a strong cooling effect.
Erythritol is also used as a noncaloric sweetener in syrups; it is used to provide sensorial profile-modifying properties with intense sweeteners; and it is also used to mask unwanted aftertastes.
Erythritol is also used as a noncariogenic sweetener in toothpastes and mouthwash solutions.
Safety
Erythritol is used in oral pharmaceutical formulations, confectionery, and food products. It is generally regarded as a nontoxic, nonallergenic, and nonirritant material. However, there has been a case report of urticaria caused by erythritol.The low molecular weight of erythritol allows more than 90% of the ingested molecules to be rapidly absorbed from the small intestine; it is not metabolized and is excreted unchanged in the urine. Erythritol has a low caloric value (0.8 kJ/g). The WHO has set an acceptable daily intake of ‘not specified’ for erythritol.
Erythritol is noncariogenic; preliminary studies suggest that it may inhibit the formation of dental plaque.
In general, erythritol is well-tolerated; furthermore, excessive consumption does not cause laxative effects. There is no significant increase in the blood glucose level after oral intake, and glycemic response is very low, making erythritol suitable for diabetics.
LD50 (mouse, IP): 8–9 g/kg
LD50 (rat, IV): 6.6 g/kg
LD50 (rat, oral): >13 g/kg
저장
Erythritol has very good thermal and chemical stability. It is nonhygroscopic, and at 25°C does not significantly absorb additional water up to a relative humidity (RH) of more than 80%. Erythritol resists decomposition both in acidic and alkaline media and remains stable for prolonged periods at pH 2–10.(10) When stored for up to 4 years in ambient conditions (20°C, 50% RH) erythritol has been shown to be stable.Purification Methods
meso-Erythritol crystallises from distilled water or absolute EtOH and is dried at 60o in a vacuum oven. It sublimes at 110o in a high vacuum. It is optically inactive. [Jeans & Hudson J Org Chem 20 1565 1955, IR: Kuhn Anal Chem 22 276 1950, Beilstein 1 IV 2807.]비 호환성
Erythritol is incompatible with strong oxidizing agents and strong bases.Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe.에리트리톨 준비 용품 및 원자재
원자재
준비 용품
에리트리톨 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Nanjing Sky Hope Tongyuan Biological Engineering Co., Ltd. | +86-0086-025-69916489 +86-18852044786 |
tongyuansales@vip.sina.com | China | 323 | 58 |
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 |
sales002@sdzschem.com | China | 3050 | 58 |
| Hengshui Haoye Chemical Co.,Ltd. | +86-2102300 +86-18632882519 |
hy@chemcoms.com | China | 256 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 |
sales@ichemie.com | China | 985 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Across Biotech Jinan Co LTD | +8613031735486 |
frank@acrossbiotech.com | China | 105 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7377 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 |
sarah@tnjone.com | China | 794 | 58 |