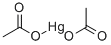아세트산 수은
|
|
아세트산 수은 속성
- 녹는점
- 179-182 °C(lit.)
- 밀도
- 3,29 g/cm3
- 증기 밀도
- 11 (vs air)
- 저장 조건
- Store at RT.
- 용해도
- 물(약간 용해됨)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Specific Gravity
- 3.27
- 색상
- 흰색에서 거의 흰색
- 냄새
- 약간의 아세트산 냄새
- 수용성
- 물에 용해됩니다.
- 감도
- Light Sensitive
- Hydrolytic Sensitivity
- 0: forms stable aqueous solutions
- Merck
- 14,5873
- BRN
- 3563831
- 안정성
- 안정적인. 강한 산화제, 강한 염기와 호환되지 않습니다. 빛에 노출되면 분해될 수 있습니다.
- CAS 데이터베이스
- 1600-27-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T+,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 26/27/28-33-50/53 | ||
| 안전지침서 | 13-28-36-45-60-61 | ||
| 유엔번호(UN No.) | UN 1629 6.1/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | AI8575000 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | II | ||
| HS 번호 | 28521000 | ||
| 유해 물질 데이터 | 1600-27-7(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-23115 | ||
| 유해화학물질 필터링 | 97-1-140 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 수은 또는 그 화합물과 수은화합물을 1% 이상 함유한 혼합물. 다만 황화 제이수은(Mercuric sulfide), 요오드화 제일수은(Mercuric iodide), 오레인산 수은(Mercuric oleate), 아미노 염화 제이수은(Amino mercury(II) chloride), 뇌산 제이수은(Mercury(II) fulminate) 및 그 중 하나를 함유한 혼합물은 제외 |
아세트산 수은 C화학적 특성, 용도, 생산
개요
Mercury (II) acetate is the chemical compound with the formula Hg(O2CCH3)2. Commonly abbreviated Hg (OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors.화학적 성질
Mercuric acetate, Hg(C2H3O2)2 , is a toxic, light-sensitive white powder, soluble in water,alcohol,and acetic acid. On exposure to heat, mercuric acetate produces toxic fumes of mercury/mercuric oxide. Mercuric acetate is incompatible with chromic acid, chromic anhydride, nitric acid, perchloric acid, permanganates, sodium peroxide, potassium hydroxide, hydrogen peroxides, acid anhydrides, and strong oxidising agents.물리적 성질
Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 ?. Three long, weak intermolecular Hg···O bonds of about 2.75 ? are also present,resulting in a slightly distorted square pyramidal coordination geometry at Hg.용도
Mercuric acetate is used as an oxidizing agent in organic synthesis. It is used in oxymercuration of double bonds. Mercuric acetate is used in non-aqueous titration. It is employed in the manufacture of phenyl mercury compounds which have pharmaceutical applications. It removes the acetamidomethyl protecting group from protected thiol, and converts thiocarbonate esters into dithiocarbonates. It promotes the addition of hydroxide and alkoxide across carbon-carbon double bonds.화학 반응
Arenes undergo "mercuration" upon treatment with Hg(OAc)2. The one acetate group that remains on mercury can be displaced by chloride :C6H5OH + Hg(OAc)2 → C6H4(OH)-2-HgOAc + HOAc
C6H4(OH)-2-HgOAc + NaCl → C6H4(OH)-2-HgCl + NaOAc
The Hg2+ center binds to alkenes, inducing the addition of hydroxide and alkoxide. For example, treatment of methylacrylate with mercuric acetate in methanol gives an α - mercuri ester :
Hg(OAc)2 + CH2 = CHCO2CH3 + CH3OH → CH3OCH2CH(HgOAc)CO2CH3+ HOAc
Mercury(II) has a high affinity for sulfur ligands. Hg (OAc)2 can be used as a reagent to remove the acetamidomethyl protecting group, which is used to "protect" thiol groups in organic synthesis. Similarly Hg(OAc)2 is a standard reagent to convert thiocarbonate esters into dithiocarbonates:
(RS)2C=S + H2O + Hg(OAc)2 → (RS)2C=O + HgS + 2 HOAc
Mercury (II) acetate is used for oxymercuration reactions.
일반 설명
White crystalline solid with an odor of vinegar. Sensitive to light. Density 3.25 g / cm3. Toxic by inhalation (dust, etc.) and by ingestion.공기와 물의 반응
Water soluble. Decomposed by water to form a yellow insoluble product.반응 프로필
MERCURIC ACETATE is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, and copper .위험도
Toxic by ingestion, inhalation, and skin absorption; strong irritant.건강위험
MERCURIC ACETATE may cause death by hypovolemic shock or kidney failure. Chronic exposure may lead to kidney failure.화재위험
When heated to decomposition, MERCURIC ACETATE emits toxic fumes of mercury. Avoid light.Safety Profile
Poison by ingestion, intravenous, intraperitoneal, and subcutaneous routes. Moderately toxic by skin contact. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Hg. See also MERCURY COMPOUNDS.잠재적 노출
Mercuric acetate is used chiefly for mercuration of organic compounds; for the absorption ofethylene; as a chemical intermediate for phenylmercuric acetate; a mildewcide; and other organomercury compounds. It is used as a catalyst in organic synthesis; and in the manufacture of pharmaceuticals.운송 방법
UN1629 Mercury acetate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Purification Methods
Recrystallise it from glacial acetic acid. POISONOUS. [Beilstein 2 IV 114.]비 호환성
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Light and heat can cause decomposition.아세트산 수은 준비 용품 및 원자재
원자재
준비 용품
아세트산 수은 관련 검색:
수은 머큐로스 아세트산 페닐수은 아세트산 아세트산 수은 수은 올레이트 수은 살리실레이트
Mercuric trifluoroacetate
MERCURY STEARATE
MERCURIC BENZOATE
FLUORESCEIN MERCURIC ACETATE,FLUORESCEIN MERCURIC ACETATE, 92%, PURE,FLUORESCEIN MERCURIC ACETATE, 96%, PURE,Fluorescein mercuric acetate, pure
Methoxyethyl mercury acetate
(2-Ethoxyethyl)mercury(II)acetate
FLUORESCEIN MERCURIC ACETATE, FOR THE DE T. OF DIS. GROUPS
METHYL MERCURIC ACETATE
MERCURY CYCLOHEXANEBUTYRATE
(4-((4-(dimethylamino)phenyl)azo)phenyl)mercuric acetate
MERCURIC CITRATE
MERCURIC ACETATE [57.1.6]