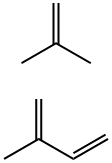
이소부틸렌-이소프렌 공중합체
|
|
이소부틸렌-이소프렌 공중합체 속성
- LogP
- 2.413 (est)
안전
| 위험 카페고리 넘버 | 10 | ||
|---|---|---|---|
| 안전지침서 | 16 | ||
| 기존화학 물질 | KE-23532 |
이소부틸렌-이소프렌 공중합체 C화학적 특성, 용도, 생산
개요
A synthetic copolymer containing from 0.5 to 2.0 molar percent of isoprene, the remainder, respectively, consisting of isobutylene. It is prepared by copolymerization of isobutylene and isoprene in methyl chloride solution, using aluminum chloride as the catalyst. After completion of polymerization, the rubber particles aretreated with hot water containing a suitable food-grade deagglomerating agent, such as stearic acid. Finally, the coagulum is dried to remove residual volatiles.화학적 성질
Butyl rubber has the chemical resistance characteristic of saturated hydrocarbons. Oxidative degradation is slow, and the rubber may be further protected by incorporating antioxidants. Isobutylene is the major component of bulk rubber. It provides good aging resistance and low gas permeability. Isoprene, a minor component, enhances vulcanizability.용도
Butyl rubber is formed from the polymerization of isobutene (isobutylene) with small amounts of chloroprene, isoprene, or butadiene and may be halogenated. The reaction is carried out in a closed system with an aluminum chloride catalyst. Butyl rubber is highly impervious to gases, which makes it the rubber best suited for inner tubes and tires, air chambers, adhesives, and dielectrics. Isobutene is an anesthetic and asphyxiant gas and can be hazardous in concentrations high enough to produce asphyxia. At these concentrations, explosion is an additional hazard.
생산 방법
The bulk of butyl rubber is made by a slurry process using aluminum chloride at 98–99°C and methyl chloride as a diluent. The extremely rapid reaction is unique, and proceeds via cationic polymerization to completion at 100°C in less than a second. Butyl rubber may be vulcanized by three basic methods: accelerated sulfur vulcanization, cross-linking with dioxime and related dinitroso compounds, and polymethylol– phenol resin cure. Halogenated butyl rubber allows broadened vulcanization latitude and rate, and enhanced covulcanization to general-purpose elastomers while it maintains the unique attributes of the basic butyl molecule.제조 방법
Commercial butyl rubbers generally contain 1-3 mole % isoprene. In a typical process, a solution of the monomers in methyl chloride is pre-cooled to -lOO??C and then fed continuously into the base of a reactor together with a solution of aluminium chloride (initiator) in the same diluent. The reaction is highly exothermic and in order to maintain the temperature at -100??C the reactor is fitted with a powerful stirrer and cooling coils containing liquid ethylene. The polymer forms as a granular slurry which is displaced continuously from the top of the reactor. The slurry passes into a flash tank where it is vigorously agitated with hot water. This treatment deactivates the initiator and extracts water-soluble products. At the same time the diluent and unreacted monomers are flashed off and are purified and re-used. Also at this point a lubricant and an antioxidant are introduced into the polymer. The lubricant, usually zinc stearate, prevents agglomeration of wet crumb and the antioxidant, usually phenyl-p-naphthylamine, reduces degradation during drying operations. The slurry then passes to a screen where wet polymer crumb is isolated. The crumb is dried at about 95-170??C, fed through an extruder and then hot milled. The object of these last operations is to compact the polymer and remove all traces of residual water.In butyl rubber the isoprene is present as random l,4-units and the copolymer thus has the following form:
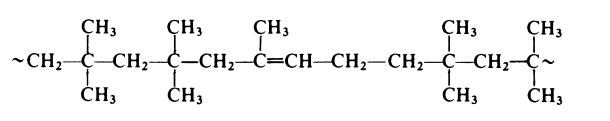
공업 용도
Butyl rubbers have outstanding impermeabilityto gases and excellent oxidation andozone resistance. The chemical inertness is furtherreflected in lack of molecular-weightbreakdown during processing, thus permittingthe use of hot-mixing techniques for betterpolymer/filler interaction.Advantages
Butyl rubber is chemically resistant to non-oxidizing dilute mineral acids, salts and alkalis, and a good chemical resistance to concentrated acids, except sulfuric and nitric acids. Moreover, it has a low permeability to air and an excellent resistance to aging and ozone. However, it is readily attacked by oxidizing chemicals, oils, benzene, and ketones and as a general rule it has also poor chemical resistance to petroleum and its derivatives and many organic chemicals. Moreover, butyl rubbers are sensitive to UV-irradiation (e.g., sunlight exposure). As with other rubbers, its mechanical properties can be largely improved by the vulcanization process.이소부틸렌-이소프렌 공중합체 준비 용품 및 원자재
원자재
준비 용품
이소부틸렌-이소프렌 공중합체 공급 업체
글로벌( 40)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7786 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | 18192627656 |
1012@dideu.com | China | 3422 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 |
info@dycnchem.com | CHINA | 52867 | 58 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 |
sales01@sdlookchemical.com | China | 2739 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 |
peter68@ptchemgroup.com | China | 35453 | 58 |
| Nanjing Bicbiotechnology Co., Ltd | +86-2552131256 +86-18251840740 |
sales@bicbiotech.com | China | 6000 | 58 |
이소부틸렌-이소프렌 공중합체 관련 검색:
이소부텐 코발트(II) 아세틸아세토네이트 하이드레이트 알루미늄 아세틸아세토네이트 실버 아세틸아세토네이트 아세틸아세토산 제2철 아세틸아세톤산 구리 디스프로슘 트리(2,2,6,6-테트라메틸-3,5-헵탄디오네이트) 이소프렌(2-메틸-1,3-부타디엔)
Benzyl isocyanide
SALCOMINE
PHENYLSELENOL
TERT-BUTYL ISOCYANIDE
Tosylmethyl isocyanide
1,1,3,3-TETRAMETHYLBUTYL ISOCYANIDE
TRIS(2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONATO)EUROPIUM(III)
N-BUTYLISOCYANIDE
Tris(2,4-pentanedionato)chroMiuM(III)
DICHLORO(ETHYLENEDIAMINE)PLATINUM(II)