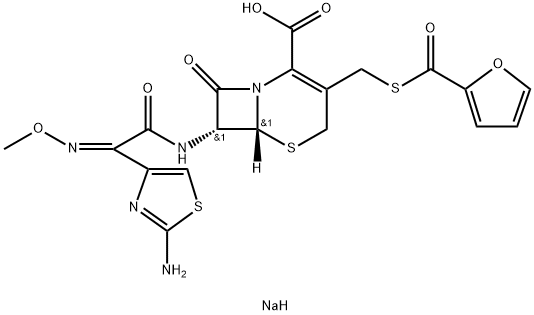
Ceftiofur sodium
|
|
Ceftiofur sodium 속성
- 녹는점
- >185°C (dec.)
- RTECS 번호
- XI0367360
- 저장 조건
- under inert gas (nitrogen or Argon) at 2–8 °C
- 용해도
- 산성 수용액에 자유롭게 용해됨
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 옅은 노랑
- InChIKey
- RFLHUYUQCKHUKS-JUODUXDSSA-M
- SMILES
- C(C1=C(CS[C@]2([H])[C@H](NC(=O)/C(/C3N=C(N)SC=3)=N\OC)C(=O)N12)CSC(C1OC=CC=1)=O)(=O)O.[NaH] |&1:5,7,r|
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| WGK 독일 | 3 | ||
|---|---|---|---|
| HS 번호 | 2941906000 |
| 그림문자(GHS): |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | ||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||||||||||||||||
| 예방조치문구: |
|
Ceftiofur sodium C화학적 특성, 용도, 생산
개요
Ceftiofur sodium is the sodium salt of ceftiofur, which belongs to the third generation cephalosporin antibiotics, for the first time in 1988 it was listed in the United States. Chemical properties are stabie, appearance is light yellow to white crystalline powder, aqueous solution has a shorter retention time, it should be diluted into a solution before use.Ceftiofur has a broad antimicrobial spectrum , it has a strong antibacterial activity, it has a strong antibacterial activity against Gram-positive bacteria, Gram-negative bacteria, and some anaerobic bacteria ,its antibacterial activity is similar to or weaker than the first-generation cephalosporins against Gram-positive bacteria, it has a strong antibacterial activity against Gram-negative bacteria such as E. coli, Salmonella, Pasteurella and so on. It is used for a variety of respiratory, urinary tract and other infections caused by sensitive bacteria, especially for the prevention and treatment of early death of chicks caused by E. coli, Salmonella, Pseudomonas aeruginosa, Staphylococcus aureus , E. coli yellow dysentery of 1 piglets and wound infections caused by cutting the umbilical cord, ear numbers, cutting teeth, tails and others,pleuropneumoniae and bovine bronchial pneumonia caused by Haemophilus and Actinobacillus, generally it does not apply to cows and goats mastitis treatment . Some researchers have done ceftiofur sodium inhibition tests for thousands strains of pathogens isolated by veterinary clinically, the results show that the drug is one of the most active anti-bacterial drugs, it has a strong antibacterial activity against Escherichia coli, p.multocida and p.haemolytica ,Bacillus typhosus, Streptococcus ; it also has a good effect on Pseudomonas aeruginosa, Enterobacter, anaerobic bacteria. It is used for the treatment of respiratory disease of sheep,horses, cattle, pigs (pleuropneumonia),and the treatment of pets, day-old broiler infectious diseases. This product has characteristics such as complete intramuscular absorption and long elimination half-life period.
The above information is edited by the chemicalbook of Tian Ye.
화학적 성질
White or light yellow crystalline powder용도
Ceftiofur sodium is resistant to the antibiotic resistance enzyme beta-lactamase, and has activity against Gram-positive and Gram-negative bacteria. E. coli strains resistant to ceftiofur have been reported. The bactericidal activity of ceftiofur results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins. The PBPs are transpeptidases which are vital in peptidoglycan biosynthesis. Therefore, their inhibition prevents this vital cell wall component from being properly synthesized.Indications
Ceftiofur sodium is a third-generation broad-spectrum cephalosporin (β-lactam antibiotic) which is effective against Gram-positive, Gram-negative, anaerobic, and β-lactamase producing bacteria. It belongs to antibiotic medicines for the treatment of swine bacterial respiratory infection.상표명
Naxcel부작용
Adverse reactions to Ceftiofur sodium are rare with intramuscular antimicrobial treatment with ceftiofur hydrochloride at a dose of 2.2 mg/kg. During the first day, the mare showed no signs of drug side effects. However, 24 hours later, during the second application, the patient developed incoordination, dizziness and loss of balance. At the same time, dexamethasone was given. Signs returned and the mare returned to normal after the reaction. Treatment with ceftiofur was changed to enrofloxacin and the animal recovered completely. These drug reactions are not common in the day-to-day work of equine clinical practitioners, but they are of great concern to owners and veterinarians[1].Veterinary Drugs and Treatments
Labeled indications for ceftiofur sodium:In cattle for treatment of bovine respiratory disease (shipping fever, pneumonia) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni. It is also indicated for treatment of acute bovine interdigital necrobacillosis (foot rot, pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus.
In swine for treatment/control of swine bacterial respiratory disease (swine bacterial pneumonia) associated with Actinobacillus (Haemophilus) pleuropneumoniae, Pasteurella multocida, Salmonella choleraesuis and Streptococcus suis.
In sheep/goats for treatment of sheep/caprine respiratory disease (sheep/goat pneumonia) associated with Mannheimia haemolytica and Pasteurella multocida.
In horses for treatment of respiratory infections in horses associated with Streptococcus zooepidemicus. In dogs for the treatment of canine urinary tract infections associated with E. coli and Proteus mirabilis.
In day old chicks/poults for the control of early mortality, associated with E. coli organisms susceptible to ceftiofur.
Ceftiofur sodium has also been used in an extra-label manner in a variety of veterinary species (see Doses) to treat infections that likely to be susceptible to a 3rd generation cephalosporin.
Ceftiofur sodium 준비 용품 및 원자재
원자재
준비 용품
Ceftiofur sodium 공급 업체
글로벌( 422)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hpets Biotech Company (Chongqing) Co.,ltd | +8613340353813 |
info@hanpets.com | China | 26 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 |
sales@sdperfect.com | China | 294 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 |
Mandy@hangyubiotech.com | China | 11013 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 |
admin@guyunchem.com | China | 616 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-13474506593 +86-13474506593 |
sarah@tnjone.com | China | 864 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |