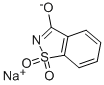
사카린나트륨
|
|
사카린나트륨 속성
- 녹는점
- >300°C
- 밀도
- 1.69[at 20℃]
- 증기압
- 0Pa at 25℃
- 저장 조건
- 0-6°C
- 용해도
- H2O: 20°C에서 1M, 투명, 무색; 에탄올에 거의 용해되지 않습니다.
- 산도 계수 (pKa)
- 0.003-0.003[at 20 ℃]
- 색상
- 백색 결정 또는 백색의 결정성 풍화분말
- 냄새
- 무취 또는 희미하고 방향족 냄새가 난다.
- 수용성
- 20°C에서 >=10g/100mL
- 안정성
- 안정적인. 강한 산화제와 호환되지 않습니다.
- InChIKey
- WINXNKPZLFISPD-UHFFFAOYSA-M
- LogP
- -2.84-0.11 at 25℃
- CAS 데이터베이스
- 128-44-9(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 안전지침서 | 24/25 | ||
|---|---|---|---|
| WGK 독일 | 2 | ||
| RTECS 번호 | DE4550000 | ||
| 독성 | LD50 oral in rat: 14200mg/kg | ||
| 기존화학 물질 | KE-02683 |
| 그림문자(GHS): |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 예방조치문구: |
|
사카린나트륨 C화학적 특성, 용도, 생산
화학적 성질
Saccharin sodium occurs as a white, odorless or faintly aromatic, efflorescent, crystalline powder. It has an intensely sweet taste, with a metallic or bitter aftertaste that at normal levels of use can be detected by approximately 25% of the population. The aftertaste can be masked by blending saccharin sodium with other sweeteners. Saccharin sodium can contain variable amounts of water.용도
Saccharin Sodium is a flavoring agent and non-nutritive sweetener. It is a salt of saccharin widely used as sweetener in food and beverage. As a high-intensity sweetener, Saccharin Sodium can be used in a wide variety of industries including: food production, beverage, cosmetics, agriculture/animal feed, and various other industries. Saccharin Sodium Salt Hydrate can be used for the purification of recombinant polypeptides, such as antibodies.생산 방법
Saccharin is produced by the oxidation of o-toluene sulfonamide by potassium permanganate in a solution of sodium hydroxide. Acidification of the solution precipitates saccharin, which is then dissolved in water at 50℃ and neutralized by addition of sodium hydroxide. Rapid cooling of the solution initiates crystallization of saccharin sodium from the liquors.일반 설명
Saccharin sodium appears as odorless white crystals or crystalline powder. Aqueous solution is neutral or alkaline to litmus, but not alkaline to phenolphthalein. Effloresces in dry air. Intensely sweet taste. (NTP, 1992)공기와 물의 반응
Water soluble.반응 프로필
Saccharin sodium may react with oxidizing agents. . Very weak base in aqueous solution.위험도
The use of saccharin is being limited due to possible carcinogenicity.화재위험
Flash point data are not available for Saccharin sodium , but Saccharin sodium is probably combustible.Pharmaceutical Applications
Saccharin sodium is an intense sweetening agent used in beverages, food products, table-top sweeteners, and pharmaceutical formulations such as tablets, powders, medicated confectionery, gels, suspensions, liquids, and mouthwashes. It is also used in vitamin preparations.Saccharin sodium is considerably more soluble in water than saccharin, and is more frequently used in pharmaceutical formulations. Its sweetening power is approximately 300–600 times that of sucrose. Saccharin sodium enhances flavor systems and may be used to mask some unpleasant taste characteristics.
Injection of saccharin sodium has been used to measure the armto- tongue circulation time.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Moderately toxic by ingestion and intraperitoneal routes. A promoter. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic fumes of SOx, Na2O, and NOx.Safety
There has been considerable controversy concerning the safety of saccharin and saccharin sodium in recent years; however, it is now generally regarded as a safe, intense sweetener. See Saccharin for further information.The WHO has set a temporary acceptable daily intake of up to 2.5 mg/kg body-weight for saccharin, including its salts.(3) In the UK, the Committee on Toxicity of Chemicals in Food, Consumer Products, and the Environment (COT) has set an acceptable daily intake for saccharin and its salts (expressed as saccharin sodium) at up to 5 mg/kg body-weight.
LD50 (mouse, oral): 17.5 g/kg
LD50 (rat, IP): 7.1 g/kg
LD50 (rat, oral): 14.2 g/kg
잠재적 노출
The information provided has to do, primarily, with the manufacturing of saccharin. Saccharin has been used as a nonnutritive sweetening agent. At one point the United States consumption pattern for all forms of saccharin has been estimated as 45% in soft drinks; 18% in tabletop sweeteners; 14% in fruits, juices, sweets, chew- ing gum, and jellies; 10% in cosmetics and oral hygiene products; 7% in drugs, such as coating on pills; 2% in tobacco; 2% in electroplating; and 2% for miscellaneous uses. Human exposure to saccharin occurs primarily through ingestion because of its use in many dietic foods and drinks and some personal hygiene products, including toothpastes and mouthwashes. The general public is exposed to saccharin, especially by persons required to reduce sugar intake.저장
Saccharin sodium is stable under the normal range of conditions employed in formulations. Only when it is exposed to a high temperature (125℃) at a low pH (pH 2) for over 1 hour does significant decomposition occur. The 84% grade is the most stable form of saccharin sodium since the 76% form will dry further under ambient conditions. Solutions for injection can be sterilized by autoclave.Saccharin sodium should be stored in a well-closed container in a dry place.
운송 방법
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz- ardous material, Technical Name Required.비 호환성
Saccharin sodium does not undergo Maillard browning.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contami- nant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal.Regulatory Status
Accepted for use as a food additive in Europe; ‘E954’ is applied to both saccharin and saccharin salts. Included in the FDA Inactive Ingredients Database (buccal and dental preparations; IM and IV injections; oral and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.사카린나트륨 준비 용품 및 원자재
원자재
염화 o-톨루엔술폰산
차아염소산나트륨
사카린
암모니아수
2-메틸벤젠설폰아마이드
이산화 황
클로로술폰산
파라-톨루엔설폰일 염화물
아질산 나트륨
무수프탈산
염산
메틸알콜
수산화나트륨
황산
준비 용품
사카린나트륨 공급 업체
글로벌( 422)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Wuhan Pinestone Fortune International Trading Co., Ltd. | +86-027-85615902 +86-13971435335 |
imp.exp8@fengfan.net | China | 29 | 58 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 |
sales@simchoicechem.com | China | 255 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 |
admin@china-yime.com | China | 563 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7786 | 58 |
| Shaanxi Franta Biotechnology Co., Ltd | +86-13082019107 +86-13082019107 |
admin@flanderff.com | China | 229 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 |
fanfan@kangcang.com.cn | China | 338 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 874 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |