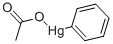페닐수은 아세트산
|
|
페닐수은 아세트산 속성
- 녹는점
- 148-151 °C(lit.)
- 밀도
- 2,4 g/cm3
- 저장 조건
- APPROX 4°C
- 용해도
- 물에 약간 용해되고 아세톤과 알코올에 용해됩니다.
- 물리적 상태
- 가루
- Specific Gravity
- 2.4
- 색상
- 하얀색
- 냄새
- 아세트산 냄새
- 수용성
- 알코올, 벤젠, 빙초산에 용해됩니다. 물에 약간 용해됩니다.
- Merck
- 14,7300
- 안정성
- 안정적인. 강한 산화제와 호환되지 않습니다.
- CAS 데이터베이스
- 62-38-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 25-34-48/24/25-50/53 | ||
| 안전지침서 | 23-24/25-37-45-60-61 | ||
| 유엔번호(UN No.) | UN 1674 6.1/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | OV6475000 | ||
| F 고인화성물질 | 8 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | II | ||
| HS 번호 | 28521000 | ||
| 유해 물질 데이터 | 62-38-4(Hazardous Substances Data) | ||
| 독성 | LD50 oral in rat: 41mg/kg | ||
| 기존화학 물질 | KE-05-1008 | ||
| 유해화학물질 필터링 | 97-1-140;06-4-16 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 수은 또는 그 화합물과 수은화합물을 1% 이상 함유한 혼합물. 다만 황화 제이수은(Mercuric sulfide), 요오드화 제일수은(Mercuric iodide), 오레인산 수은(Mercuric oleate), 아미노 염화 제이수은(Amino mercury(II) chloride), 뇌산 제이수은(Mercury(II) fulminate) 및 그 중 하나를 함유한 혼합물은 제외 |
페닐수은 아세트산 C화학적 특성, 용도, 생산
화학적 성질
Phenylmercuric acetate occurs as a white to creamy white, odorless or almost odorless, crystalline powder or as small white prisms or leaflets.용도
Phenylmercuric acetate is used as catalyst; fungicide; herbicide; algicide; preservative in antibiotic eye drops, eye cosmetics, shampoos, etc.생산 방법
Phenylmercuric acetate is readily formed by heating benzene with mercuric acetate.일반 설명
Small lustrous prisms. Toxic by ingestion, inhalation and skin absorption. May severely irritate skin and eyes. Used as an herbicide and fungicide. as such, is mixed with organic solvent for the purpose of application.반응 프로필
PHENYLMERCURIC ACETATE may react with strong oxidizing agents .건강위험
Extremely toxic. The probable oral lethal dose for humans is 5-50 mg/kg, between 7 drops and 1 teaspoonful for a 70 kg (150 lb.) person.화재위험
Fire may produce irritating or poisonous gases. When heated to decomposition, very toxic mercuric fumes may be given off. Phenylmercuric ion is incompatible with halides, with which precipitates are formed.Pharmaceutical Applications
Phenylmercuric acetate is used as an alternative antimicrobial preservative to phenylmercuric borate or phenylmercuric nitrate in a limited range of cosmetics (in concentrations not exceeding 0.007% of mercury calculated as the metal) and pharmaceuticals. It may be used in preference to phenylmercuric nitrate owing to its greater solubility.Phenylmercuric acetate is also used as a spermicide;
Safety Profile
Poison by ingestion, intravenous, intraperitoneal, subcutaneous, and possibly other routes. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. See also MERCURY COMPOUNDS. When heated to decomposition it emits toxic fumes of Hg.Safety
Phenylmercuric acetate is mainly used as an antimicrobial preservative in topical pharmaceutical formulations. A number of adverse reactions to mercury-containing preservatives have been reported; see Phenylmercuric Nitrate.LD50 (chicken, oral): 60 mg/kg
LD50 (mouse, IP): 13 mg/kg
LD50 (mouse, IV): 18 mg/kg
LD50 (mouse, oral): 13 mg/kg
LD50 (mouse, SC): 12 mg/kg
LD50 (rat, oral): 41 mg/kg
잠재적 노출
Phenylmercury acetate is used as an antiseptic, fungicide; for fungal and bacterial control; herbicide and control of crabgrass; mildewcide for paints; slimicide in paper mills. It was also used in contraceptive gels and foams.환경귀착
If released into air, soil, or water, phenylmercuric acetate is unlikely to volatilize and is instead expected to be bound to particulates based on a low vapor pressure (6 × 10-6 mm Hg) and low Henry’s constant (5.66 × 10-10 atmm3 mol -1). Photolysis has the potential to degrade phenylmercuric acetate, releasing inorganic mercury which can volatilize and enter the atmosphere from superficial soils or water. If released into soil, the mobility of parent phenylmercuric acetic acid is expected to be high based on a Koc of 60. Water releases would result in quick dispersion since water solubility is high (4370 mg l-1). Once in solution, especially with harder water, it will dissociate into a salt. The cation will adsorb to particulates or humics suspended in the water column or in sediment, with little bioconcentration in aquatic species.저장
As for other phenylmercuric salts; see Phenylmercuric Nitrate.Phenylmercuric acetate should be stored in a well-closed container, protected from light, in a cool, dry place.
운송 방법
UN1674 Phenylmercuric acetate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Purification Methods
It forms small colourless lustrous prisms from EtOH. Its solubility in H2O is 0.17%, but it is more soluble in EtOH, Me2CO and *C6H6. [Maynard J Am Chem Soc 46 1510 1925, Coleman et al. J Am Chem Soc 59 2703 1937, J Am Pharm Assoc 25 752 1936, Beilstein 16 IV 1720.] See PhHgOH below.비 호환성
As for other phenylmercuric salts; see Phenylmercuric Nitrate.Incompatible with: halides; anionic emulsifying agents and suspending agents; tragacanth; starch; talc; sodium metabisulfite; sodium thiosulfate; disodium edetate; silicates; aluminum and other metals; amino acids; ammonia and ammonium salts; sulfur compounds; rubber; and some plastics.
Phenylmercuric acetate is reported to be incompatible with cefuroxime and ceftazidime.
폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. React to produce soluble nitrate form, precipitate as mercuric sulfide. Return to supplier.Regulatory Status
Included in the FDA Inactive Ingredients Database (ophthalmic ointments; topical emulsions/creams; vaginal emulsions/creams). Included in the Canadian List of Acceptable Non-medicinal Ingredients (ophthalmic, nasal and otic preparations up to 0.004%; there must be no other suitable alternative preservative available).Phenylmercuric acetate is no longer permitted to be used as a pesticide in the USA. Its use in cosmetic products in the USA is limited to eye area cosmetics at not more than 0.0065% provided that there is no other suitable available preservative. It is specifically prohibited in vaginal contraceptive drug products and antimicrobial diaper rash drug products in the USA. Phenylmercuric compounds are prohibited from use in cosmetic products in Canada.
In Europe, use in cosmetic products is limited to eye makeup and eye makeup remover at concentrations not exceeding 0.007% mercury alone or in combination with other permitted mercurial compounds.In France, a maximum concentration of 0.01% is permitted for use in pharmaceuticals. The use of mercurial compounds in cosmetics in Japan is limited to concentrated shampoo or cream at not more than 0.003% Hg and eye makeup at not more than 0.0065% Hg.
페닐수은 아세트산 준비 용품 및 원자재
원자재
준비 용품
페닐수은 아세트산 관련 검색:
아세트산 수은 수은 페닐수은 아세트산
Phenylmercurypropionate
acetomeroctol
PHENYL MERCURIC BENZOATE
FLUORESCEIN MERCURIC ACETATE
1-(4-ACETOXYMERCURIPHENYLAZO)-2-NAPHTHOL
P-AMINOPHENYLMERCURIC ACETATE
4,5-DIMETHYL-3,6-DIOCTYLOXY-1,2-PHENYLENE-BIS(MERCURY TRIFLUOROACETATE)
PHENYLMERCURIC OLEATE
FLUORESCEIN MERCURICACETATE FOR THE
PHENYLMERCURY SALICYLATE
(LACTOYLOXY)PHENYLMERCURY
PHENYL MERCURIC PHTHALATE
Tolylmercuric acetate
PHENYLMERCURIC ACETATE - REFERENCE SPECTRUM
PHENYLMERCURIC STEARATE