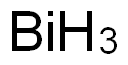비스무트
|
|
비스무트 속성
- 녹는점
- 271 °C (lit.)
- 끓는 점
- 1560 °C (lit.)
- 밀도
- 9.8 g/mL at 25 °C (lit.)
- 증기압
- <0.1 mm Hg ( 20 °C)
- 저장 조건
- no restrictions.
- 용해도
- soluble in acid solutions
- Specific Gravity
- 9.80
- 색상
- 은백색 또는 빨간색
- 비저항
- 129 μΩ-cm, 20°C
- 수용성
- 불용성
- Merck
- 13,1256
- 노출 한도
- ACGIH: TWA 2 ppm; STEL 4 ppm
OSHA: TWA 2 ppm(5 mg/m3)
NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3)
- 안정성
- 안정적인. 강산, 강산화제, 니트로실 플루오라이드, 용융 질산암모늄, 할로겐간 화합물, 염소와 호환되지 않습니다. 미세하게 분할된 분말은 가연성이 높습니다.
- CAS 데이터베이스
- 7440-69-9(CAS DataBase Reference)
- NIST
- Bismuth(7440-69-9)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,C,Xi,O | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-11-36/37/38-8 | ||
| 안전지침서 | 16-45-36/37/39-26 | ||
| 유엔번호(UN No.) | UN 3264 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | EB2600000 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 81060010 | ||
| 유해 물질 데이터 | 7440-69-9(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-03313 |
비스무트 C화학적 특성, 용도, 생산
화학적 성질
Bismuth is the fifth element in the nitrogen group, and its properties are the most metal-likeof the five. Elemental bismuth is a heavy, brittle, hard metal that can be polished to a brightgray-white coat with a pinkish hue. It is not found in this state very often because it is more likelyto be combined with other metals and minerals, such as tin, lead, iron and cadmium. These aremixtures with low melting points, making them useful in fire-detection devices.When heated in air, bismuth burns with a blue flame, giving off clouds of its yellow oxide.Bismuth’s melting point is 271.40°C, its boiling point is 1,564°C, and its density is 9.807 g/cm3.
물리적 성질
Bismuth has unusually low toxicity for a heavy metal. Bismuth is stable to both dry and moist air at ordinary temperatures. When red hot, it reacts with water to make bismuth(III) oxide. Bismuth forms trivalent and pentavalent compounds. The trivalent compounds are more common. Many of its chemical properties are similar to those of As and Sb, although they are less toxic than derivatives of those lighter elements. At elevated temperatures, the vapors of the metal combine rapidly with oxygen, forming the yellow trioxide, Bi2O3.Isotopes
There are a total of 59 radioactive isotopes for bismuth, ranging in half-livesfrom a few milliseconds to thousands of years. At one time it was thought that there wasjust one stable isotope (Bi-209), but it was later found that Bi-209 is radioactive witha half-life of 19,000,000,000,000,000,000 years. Such a long half-life means that Bi-209 has not completely disintegrated and is still found in nature, and is thus consideredstable. In this case, Bi-209 makes up 100% of Bismuth’s natural abundance.Origin of Name
Bismuth was known and used by the ancient alchemists along with other metals both for chemical reactions and for medical purposes. The name comes from the German bismu, which had been changed from wismu, meaning “white.”출처
Bismuth is the 70th most abundant element, and it is widely spread over the Earth’s crust, butin very small amounts. There are no major concentrated sources. It occurs both in the free elementalstate and in several ores. The major ore, bismuthinite (B2S3), is found in South America.The United States gets most of its bismuth as a by-product from smelting ores of lead,silver, copper, and gold. It is also recovered from the refining of tin and tungsten ores.
Characteristics
Bismuth is more resistant to electrical current in its solid state than it is in its liquid form.Its thermal conductivity is the lowest of all metals, except mercury. Even though it is considereda metal-like element, it is a very poor conductor of heat and electricity.Bismuth has a characteristic similar to water. It expands when changing from the liquidphase to the solid phase. This factor makes it useful as an alloy in metals that are used to fillmolds, given that it will expand to the cast’s dimensions.
용도
Bismuth is used to make the drugs such as Pepto-Bismol for upset stomachs and diarrheaand in medicine to treat intestinal infections. Bismuth is used in the cosmetics industry toprovide the “shine” for lipsticks, eye shadow, and other products.It is added to steel and other metals as an alloy to make the metals easier to roll, press, pullinto wires, and turn on a lathe. It is also used in the semiconductor industry and to makepermanent magnets.
Bismuth is similar to antimony in that it expands from the molten liquid state to the solidstate. This property makes it an excellent material to pour into molds and can produce finedetails in whatever is being molded, such as metallic printing type and similar fine castings.
정의
A brittle pinkish metallic element belonging to group 15 (formerly VA) of the periodic table. It occurs native and in the ores Bi2S3 and Bi2O3. The element does not react with oxygen or water under normal temperatures. It can be dissolved by concentrated nitric acid. Bismuth is widely used in alloys, especially low-melting alloys. The element has the property of expanding when it solidifies. Compounds of bismuth are used in cosmetics and medicines.일반 설명
All foils are mounted on a permanent support which cannot be removed without damaging the foil.위험도
Bismuth is flammable as a powder. The halogen compounds of bismuth are toxic wheninhaled or ingested. Some of the salts of bismuth can cause metallic poisoning in a mannersimilar to mercury and lead.At the beginning of the twentieth century, before penicillin, bismuth compounds wereused to treat some venereal diseases. However, the treatment was generally unsuccessful.
건강위험
Exposures to bismuth salts are associated primarily by ingestion. Bismuth is known to cause adverse health effects. The symptoms include, but are not limited to, irritation of the eyes, skin, respiratory tract, lungs, foul breath, metallic taste, and gingivitis. On ingestion, bismuth causes nausea, loss of appetite, weight, malaise, albuminuria, diarrhea, skin reactions, stomatitis, headache, fever, sleeplessness, depression, rheumatic pain, and a black line may form on gums in the mouth due to deposition of bismuth sulfi de. Prolonged exposure to bismuth causes mild but deleterious effects on the kidneys and high concentrations of bismuth result in fatalities. Occupational exposures to bismuth occur during the manufacture of cosmetics, industrial chemicals, and pharmaceuticals. Acute exposure with over dosage of bismuth-containing drugs causes anorexia, nausea, vomiting, abdominal pain, and possibly a dry mouth and thirst. Bismuth also causes neurotoxicity. Bismuth pentafl uoride is highly toxic and causes irritation to the skin, eyes, and respiratory tract, while bismuth subnitrate causes blurred vision.잠재적 노출
Bismuth is used as a constituent of tempering baths for steel alloys; in low Freezing/Melting point alloys which expand on cooling; in aluminum and steel alloys to increase machinability; and in printing type metal. Bismuth compounds are found primarily in pharmaceuticals as antiseptics, antacids, antiluetics, and as a medicament in the treatment of acute angina. They are also used as a contrast medium in roentgenoscopy and in cosmetics. For the general population the total intake from food is 5 20 μg with much smaller amounts contributed by air and water.Carcinogenicity
An old lifetime study with rats fed 2% bismuth subcarbonate (BSC) in the diet did not show an increase of tumors or a decrease of survival.환경귀착
The mechanism by which bismuth produces toxicity has not been identified. Interaction with thiol compounds has been proposed as a primary mechanism.운송 방법
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1—Flammable solid.Purification Methods
Melt it in an atmosphere of dry helium, then filter through dry Pyrex wool to remove any bismuth oxide present [Mayer et al. J Phys Chem 64 238 1960].Structure and conformation
The space lattice of Bi belongs to the trigonal system, and its arsenic type structure has lattice constants of a=0.4736 nm, Bi–Bi=0.310 nm, α=57°16', and u=0.474.비 호환성
Finely divided powder is highly flammable. Reacts with strong acids and strong oxidizers, chlorine, fused ammonium nitrates, iodine pentafluoride, and nitrosyl fluoride.폐기물 처리
Dissolve in a minimum amount of concentrated HCl. Dilute with water until precipitate is formed. Redissolve in HCl. Then saturate with H2S. Filter, wash, dry and return to supplier.비스무트 준비 용품 및 원자재
원자재
준비 용품
비스무트 공급 업체
글로벌( 351)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 |
admin@hbsaisier.cn | China | 690 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 |
eric@witopchemical.com | China | 23556 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 9358 | 58 |