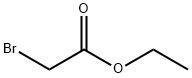
에틸브로모아세트산 C화학적 특성, 용도, 생산
물성
물에 녹지 않는다. 수산화나트륨 용액에 의해 가수 분해된다. 철과 반응한다. 눈을 강하게 자극함과 동시에 유독성이다.
용도
1) 유기 합성 원료.
2) 독가스(제1차 세계 대전에 최초로 사용된 독가스로, 염소를 사용한 1915년 4월에 앞서 1914년 말에 프랑스군이 가스 유탄으로 사용했다고 한다).
화학적 성질
Ethyl bromoacetate is a clear, colorless to light-yellow liquid and has a pungent odor. It is miscible with ethanol, ether, benzene and with other oxygenated and aromatic solvents, insoluble in water and partially decomposed by water.
주요 응용
Ethyl Bromoacetate is used as a synthetic organic chemical intermediate as well as a pharmaceutical and agricultural intermediate. It is used in the synthesis of metabolites of carcinogenic PAHs. It is also used in the preparation of steroidal antiestrogens through cyclic condensation. Ethyl Bromoacetate is a reactant in the preparation of antim icrobial and antioxidant coumarinyloxymethyl-thiadiazolone.
제조 방법
Ethyl bromoacetate is commonly synthesized by sulfuric acid catalyzed esterification of monobromoacetic acid (MBAA). After reaction completion, excess acid is washed out and the product purified via distillation under reduced pressure if necessary (Stenger, 1978; Korhonen, 1984).
화학 반응
Ethyl bromoacetate on derivatisation reaction with p-t-butyl calix[4]arene yields 1,3-diester substituted calix[4]arene. It also undergoes Suzuki type cross-coupling reactions with arylboronic acids cocatalyzed by copper(I) oxide.
일반 설명
Ethyl bromoacetate appears as a clear, colorless liquid. A lachrymator. Toxic by ingestion, inhalation and skin absorption; a strong irritant of the skin. Insoluble in water and soluble in alcohol, benzene, and ether. Specific gravity of 1.5.
공기와 물의 반응
Flammable. Insoluble in water.
반응 프로필
Ethyl bromoacetate is a halogenated ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
위험도
Toxic by ingestion, inhalation, and skin
absorption; strong irritant.
건강위험
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Bromoacetates and chloroacetates are extremely irritating/lachrymators. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
화재위험
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
A poison. An irritant to
skin, eyes, and mucous membranes.
Questionable carcinogen with experimental
neoplastigenic data. Flammable liquid when
exposed to heat, flame, and oxidizers. Will
react with water or steam to produce toxic
and corrosive fumes. To fight fire, use water
as a fire blanket. When heated to
decomposition or on contact with acid or
acid fumes, it emits highly toxic fumes of
Br-. See also BROMIDES.
잠재적 노출
Used for making pharmaceuticals; as
a warning gas in poisonous, odorless gasses; as a tear gas
운송 방법
UN1603 Ethyl bromoacetate, Hazard class: 6.1;
Labels: 6.1-Poisonous materials.
Purification Methods
Wash the ester with saturated aqueous Na2CO3 (three times), 50% aqueous CaCl2 (three times) and saturated aqueous NaCl (twice). Dry with MgSO4, CaCl2 or CaCO3, and distil it. [Beilstein 2 IV 527.] LACHRYMATORY.
비 호환성
Vapor may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides, and reducing agents. Esters are generally incompatible with nitrates. Moisture may cause hydrolysis or other forms of decomposition.
에틸브로모아세트산 준비 용품 및 원자재
원자재
준비 용품
ETHYL(5-BROMOBENZOFURAN)-2-CARBOXYLATE
ETHYL 3-AMINOBENZOFURAN-2-CARBOXYLATE
5-BROMO-1-BENZOFURAN-2-CARBALDEHYDE
3-METHYLBENZOFURAN-2-CARBONYL CHLORIDE
ethyl 2-(2-aminothiazol-5-ylthio)acetate
3-METHYLBENZOFURAN-2-CARBOXYLIC ACID ETHYL ESTER
3-O-TOLYL-PROPIONALDEHYDE
Ethyl 2-oxocyclopentylacetate
5-BROMO-1-BENZOFURAN-2-CARBOXYLIC ACID
2-Phenyl-1H-indole-1-acetic acid ,97%
(R)-3-Aminoquinuclidine dihydrochloride
3,4-DIMETHYLTHIENO[2,3-B]THIOPHENE-2-CARBALDEHYDE
(5-BROMO-1-BENZOFURAN-2-YL)METHANOL
3-(4-CHLORO-PHENYL)-PROPIONALDEHYDE
3,5-DIMETHYL-BENZOFURAN-2-CARBOXYLIC ACID
2-Piperazinone
Rhodanine-3-acetic acid
3-CHLOROPHENOXYACETYL CHLORIDE
ETHYL METHANESULFONYLACETATE
6-BROMO-1-BENZOFURAN-2-CARBOXYLIC ACID
4-(TRIFLUOROMETHYL)PHENOXYACETIC ACID
(4-METHYL-PIPERAZIN-1-YL)-ACETIC ACID
METHYL5,5,5-TRIFLUORO-4-OXOPENTANOATE
(4-PHENYL-PIPERAZIN-1-YL)-ACETIC ACID
옥텐(트란스-2-)-1-올
2-METHYLCYCLOPROPANECARBOXYLIC ACID
MORPHOLINE-4-CARBOTHIOAMIDE
(S)-(+)-3-Quinuclidinol
Ethyl(4-butyry-2,3-dichloro)phenoxyacetate
Ethyl 3,5-dimethylbenzofuran-2-carboxylate ,95%
4-CHLORO-8-FLUORO-5H-PYRIMIDO[5,4-B]INDOLE
(4-FLUOROPHENOXY)ACETYL CHLORIDE
2-(CARBOXYMETHOXY)BENZOIC ACID
ethyl 2-(2-phenyl-1H-indol-1-yl)acetate
디에톡시프로피온산에틸에스테르
3-COUMARANONE
ETHYL 2-(5-FORMYL-2,4-DIMETHYL-1H-PYRROL-3-YL)ACETATE
quinuclidine-3-carboxylic acid
제라닉애씨드
(R)-3-Quinuclidinol hydrochloride