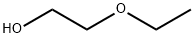에틸렌글리콜 모노에틸 에테르
|
|
에틸렌글리콜 모노에틸 에테르 속성
- 녹는점
- -100 °C
- 끓는 점
- 135 °C(lit.)
- 밀도
- 0.93 g/mL at 25 °C(lit.)
- 증기 밀도
- 3.1 (vs air)
- 증기압
- 3.8 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.407(lit.)
- 인화점
- 107.6 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 물: 섞일 수 있음
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- 14.44±0.10(Predicted)
- 색상
- 투명, 무색
- 냄새
- 달콤한; 온화하고, 쾌적하고, 미묘합니다.
- Odor Threshold
- 0.58ppm
- 폭발한계
- 1.8-15.7%(V)
- 수용성
- 혼용 가능
- 어는점
- -70℃
- 최대 파장(λmax)
- λ: 215 nm Amax: 1.00
λ: 225 nm Amax: 0.50
λ: 250 nm Amax: 0.20
λ: 305 nm Amax: 0.01
- Merck
- 14,3750
- BRN
- 1098271
- 노출 한도
- TLV-TWA skin 5 ppm (18.5 mg/m3) (ACGIH). .
- Dielectric constant
- 29.6(24℃)
- 안정성
- 안정적인. 공기와 쉽게 폭발성 혼합물을 형성합니다. 폭발 범위가 넓습니다. 증기는 발화원까지 상당한 거리를 이동할 수 있습니다. 가연성. 강산, 강염기, 강산화제, 알루미늄, 알칼리와 호환되지 않습니다.
- LogP
- 0.32 at 20℃
- CAS 데이터베이스
- 110-80-5(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 60-61-10-20/21/22-20/22 | ||
| 안전지침서 | 53-45 | ||
| 유엔번호(UN No.) | UN 1171 3/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | KK8050000 | ||
| 자연 발화 온도 | 460 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| HS 번호 | 29094990 | ||
| 유해 물질 데이터 | 110-80-5(Hazardous Substances Data) | ||
| 독성 | Acute oral LD50 for guinea pigs 1,400 mg/kg, mice 2,451 mg/kg, rats 3,000 mg/kg, rabbits 3,100 mg/kg (quoted, RTECS, 1985). | ||
| IDLA | 500 ppm | ||
| 기존화학 물질 | KE-13667 | ||
| 유해화학물질 필터링 | 2014-1-696 | ||
| 중점관리물질 필터링 | 별표1-63 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 2-에톡시에탄올 및 이를 0.3% 이상 함유한 혼합물 |
에틸렌글리콜 모노에틸 에테르 C화학적 특성, 용도, 생산
용도

준비
개요
2-Ethoxyethanol is a stable, colorless, flammable liquid, synthetically produced throughout the world. It belongs to a larger group of glycol ether solvents. 2-Ethoxyethanol is commercially referred to as Ethyl Cellosolve or Cellosolve, a trademark registered by Union Carbide in 1924. It was first synthesized to have the same chemical properties of both alcohols and ethers (hydrophilic and lipophilic) but less volatile, which improves production characteristics. The glycol ethers are made by reacting anhydrous alcohols with ethylene oxide.화학적 성질
2-Ethoxyethanol is a colorless, viscous liquid with a sweetish odor물리적 성질
Clear, colorless liquid with a sweetish odor. Experimentally determined detection and recognition odor threshold concentrations were 1.1 mg/m3 (300 ppbv) and 2.0 mg/m3 (540 ppbv), respectively (Hellman and Small, 1974).용도
2-Ethoxyethanol is widely used as an industrial solvent and production intermediate. It is produced by the reaction of ethylene oxide with ethanol. The glycol ethers are miscible in polar and nonpolar solutions, which make them useful solvents in paints and surface coatings, stains, lacquers, inks, and dyes. Additional uses include industrial deicing, hydraulic fluids, and cleaning agents. 2-Ethoxyethanol was once used in cosmetic products but is no longer used due to toxicity associated with dermal absorption. Global production has been on the decline in recent years based on demonstrated toxicity through oral, dermal, and inhalation routes of exposure. The use of ethylene glycol ethers has largely been replaced by relatively safer substitutes, primarily propylene glycol ethers; however, their use as a solvent and chemical process intermediate poses potential for release into the environment.정의
ChEBI: A hydroxyether that is the ethyl ether derivative of ethylene glycol.일반 설명
A clear colorless liquid. Flash point of 120°F. Less dense than water. Its vapors are heavier than air.공기와 물의 반응
Flammable. Water soluble.반응 프로필
ETHYLENE GLYCOL MONOMETHYL ETHER may react with oxidizing materials, i.e. hydrogen peroxide, to form peroxides. 2-Ethoxyethanol dissolves many oils, resins and waxes.위험도
Toxic by skin absorption. Moderate fire risk.건강위험
Some eye irritation. Inhalation of vapors causes irritation of nose.화재위험
Special Hazards of Combustion Products: Toxic gases, such as carbon monoxide, may be produced in fire.화학 반응
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Safety Profile
Moderately toxic by ingestion, skin contact, intravenous, and intraperitoneal routes. Mildly toxic by inhalation and subcutaneous routes. An experimental teratogen. Other experimental reproductive effects. A mild eye and skin irritant. Combustible when exposed to heat or flame; can react with oxidzing materials. Moderate explosion hazard in the form of vapor when exposed to heat or flame. Mxture with hydrogen peroxide + polyacrylamide gel + toluene is explosive when dry. To fight fire, use alcohol foam, dry chemical. See also GLYCOL ETHERS.잠재적 노출
This material is used as a solvent for nitrocellulose and alkyd resins in lacquers; as a solvent for printing inks; in dyeing leathers and textiles; in the formulation of cleaners and varnish removers; as an anti-icing additive in brake fluids and auto and aviation fuels.환경귀착
Biological. Bridié et al. (1979) reported BOD and COD values of 1.03 and 1.92 g/g using filtered effluent from a biological sanitary waste treatment plant. These values were determined using a standard dilution method at 20 °C for a period of 5 d. When a sewage seed was used in a separate screening test, a BOD value of 1.27 g/g was obtained. Similarly, Heukelekian and Rand (1955) reported a 5-d BOD value of 1.42 g/g which is 72.4% of the ThOD value of 1.96 g/g.Photolytic. Grosjean (1997) reported a rate constant of 1.87 x 10-11 cm3/molecule?sec at 298 K for the reaction of 2-ethoxyethanol and OH radicals in the atmosphere. Based on an atmospheric OH radical concentration of 1.0 x 106 molecule/cm3, the reported half-life of methanol is 0.35 d (Grosjean, 1997). Stemmler et al. (1996) reported a rate constant of 1.66 x 10-11 cm3/molecule?sec for the OH radical-initiated oxidation of 2-ethoxyethanol in synthetic air at 297 K and 750 mmHg. Major reaction products identified by GC/MS (with their yields) were ethyl formate, 34%; ethylene glycol monoformate, 36%; ethylene glycol monoacetate, 7.8%; and ethoxyacetaldehyde, 24%.
Chemical/Physical. 2-Ethoxyethanol will not hydrolyze (Kollig, 1993).
At an influent concentration of 1,024 mg/L, treatment with GAC resulted in an effluent concentration of 886 mg/L. The adsorbability of the carbon used was 28 mg/g carbon (Guisti et al., 1974).
운송 방법
UN1171 Ethylene glycol monoethyl ether, Hazard Class: 3; Labels: 3-Flammable liquid.Purification Methods
Dry it with CaSO4 or K2CO3, filter and fractionally distil it. Peroxides can be removed by refluxing with anhydrous SnCl2 or by filtration under slight pressure through a column of activated alumina. [Beilstein 1 IV 2377.]비 호환성
May form explosive mixture with air. Strong oxidizers may cause fire and explosions. Attacks some plastics, rubber and coatings. Able to form peroxides. Incompatible with strong acids; aluminum and its alloys폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal에틸렌글리콜 모노에틸 에테르 준비 용품 및 원자재
원자재
준비 용품
Leather Coating Black RL
디펜아마이드
1-(2-ETHOXYETHYL)PIPERAZINE
sodium [2,4-dihydro-4-[(2-hydroxy-5-nitrophenyl)azo]-5-methyl-2-phenyl-3H-pyrazol-3-onato(2-)][1-[(2-hydroxy-4-nitrophenyl)azo]-2-naphtholato(2-)]cobaltate(1-)
9-ETHYLGUANINE
C.I. 산 흑 63
Acid Orange 89
LEATHERSPRAYINGBLUERL
에틸셀로솔브아세테이트
6-CHLOROQUINAZOLIN-4-AMINE
nitrilon whitening agent AD
2-브로모에틸에틸에테르
C.I. 산성 황색 059
2-메틸-1,3-디옥솔란
에틸렌글리콜 모노에틸 에테르 공급 업체
글로벌( 540)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12453 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8822 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |
에틸렌글리콜 모노에틸 에테르 관련 검색:
모노에틸렌글리콜 에틸셀로솔브아세테이트 옥틸페녹시폴리(에톡시에탄올) 디에틸에테르 디에틸렌 글리콜 모노에틸 에테르 아세테이트 에틸디글리콜 에톡시디에틸렌 글리콜아크릴레이트 2-(2-(2-에톡시에톡시)에톡시)에탄올 디에틸아미노에톡시에탄올
1,2-Dimethoxyethane
2-Methoxyethanol
2-Butoxyethanol
Ethylene glycol diphenyl ether
Ethylene glycol diethyl ether
Ethylene Glycol Dibutyl Ether
ETHYLENE GLYCOL MONO-TERT-BUTYL ETHER
2-(HEXYLOXY)ETHANOL
ACETIC ACID 2-PHENOXYETHYL ESTER