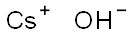수산화 세슘 C화학적 특성, 용도, 생산
화학적 성질
Cesium hydroxide is a colorless-to-yellow
crystalline compound. It is often used in a water solution.
물리적 성질
White to yellowish fused crystalline mass; highly deliquescent; very alkaline; density 3.68 g/cm
3; melts 272°C; highly soluble in water; soluble in ethanol; aqueous solution is very alkaline.
용도
Cesium hydroxide is used in the preparation of porous cesium titanosilicates, which are potential ion-exchange materials utilized for radioactive wastes cleaning purpose. It is also involved in the preparation of other cesium salts. Further, it is used as an electrolyte in alkaline storage batteries and as a catalyst in organic synthesis. In addition to this, it is used in color photography.
주요 응용
Cesium hydroxide also exerts unusual effects in organic reactions, such as the controlled alkylation of amines. In this instance, the cesium ion itself is explicitly involved in the reaction. The hydroxide base promotes an alkylation of a primary amine, while the Cs+ ion weakly coordinates to the amine such that further alkylation is inhibited, preventing formation of dialkyl and trialkyl amines.
Cesium hydroxide solution is used in industrial catalysis. Cesium acts as apromoter e.g. in the production of polyols. Polyols are important raw materials for the production of polyurethane foams. Their synthesis profits from use of cesium hydroxide as base catalyst. In polyol synthesis isomerization of the starting material propylene oxide is an adverse side reaction eventually leading to chain termination in the polymerization with polyisocyanate. The tendency of polyols to undergo isomerization is minimized when using cesium hydroxide, as isomerization tendency decreases in the order Li > Na > K > Rb > Cs.
제조 방법
Cesium hydroxide is prepared by electrolysis of cesium salts to obtain cesium metal, which then reacts with water to yield hydroxide. It also is prepared by the action of barium hydroxide with an aqueous solution of cesium sulfate.
정의
ChEBI: Caesium hydroxide is an alkali metal hydroxide and a caesium molecular entity.
일반 설명
Cesium hydroxide is a colorless to yellow crystalline solid. Harmful to skin and eyes. Used in electric storage batteries.
공기와 물의 반응
Dilution with water may generate enough heat to cause steaming or spattering.
반응 프로필
CESIUM HYDROXIDE,SOLUTION neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose, to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995].
위험도
A poison. Skin, eye, and upper respiratory
tract irritant.
건강위험
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Poison by
intraperitoneal route. Moderately toxic by
ingestion. A powerful caustic. A corrosive
skin and eye irritant. See also CESIUM.
잠재적 노출
Cesium hydroxide may be used as a
raw material for other cesium salts; such as the chloride
which in turn may be used to produce cesium metal.
Cesium metal is used in electronic devices.
운송 방법
UN2682 Cesium hydroxide, Hazard class: 8;
Labels: 8-Corrosive material. UN2681 Cesium hydroxide,
solution, Hazard class: 8; Labels: 8-Corrosive material
비 호환성
Cesium hydroxide is the strongest base
known. Keep away from all acids. It must be stored in silver or platinum and out of contact with air because of its
reactivity with glass. CsOH causes the generation of considerable heat in contact with water or moisture. Contact
with many organic compounds, many metals (i.e., aluminum, lead, tin, zinc), glass, oxygen, or carbon dioxide
causes a violent reaction.
수산화 세슘 준비 용품 및 원자재
원자재
준비 용품