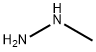
메틸히드리진 C화학적 특성, 용도, 생산
개요
Methyl hydrazine, CH3NHNH2, is a colorless, hygroscopic liquid with an ammonia-like odor. It is soluble in water, with a specific gravity of 0.87, which is lighter than water. Methyl hydrazine is toxic by inhalation and ingestion, and is a suspected human carcinogen. The TLV ceiling is 0.2 ppm in air, and the IDLH is 50 ppm. The target organs are the central nervous system, respiratory system, liver, blood, eyes, and cardiovascular system. The four-digit UN identification number is 1244. The NFPA 704 designation is health 4, flammability 3, and reactivity 2. The primary uses are as a missile propellant and a solvent.
화학적 성질
colourless liquid with an ammonia-like odour
물리적 성질
Fuming, clear, colorless liquid with an ammonia-like odor. Odor threshold concentrations ranged
from 1 to 3 ppm (quoted, Keith and Walters, 1992).
용도
Methylhydrazine is used in missile propellants and as a
solvent and chemical intermediate.
생산 방법
Methylhydrazine ignites spontaneously on contact with
strong oxidizing agents. It is prepared commercially from
the reaction of monochloroamine and monomethylamine.
공기와 물의 반응
Highly flammable. Often ignites spontaneously. Exposure to air on a large surface may result in spontaneous ignition [Def. Res. and Eng. 27. 1963]. Water soluble. Solutions are highly alkaline and generate heat when water is added.
반응 프로필
Methylhydrazine is a powerful reducing agent. Ignites upon contact with oxidizing agents i.e. dinitrogen tetraoxide, hydrogen peroxide [Hawley]. Water used to extinguish a fire may cause pollution and should be diked for later disposal. Gives basic solutions with water that generate heat when water is added.
위험도
Flammable, dangerous fire risk, vapors mayexplode, may self-ignite in air and on contact withoxidizing agents. Toxic by ingestion and inhalation.Eye and upper respiratory tract irritant, lung cancerand liver damage. Possible carcinogen.
건강위험
Methyl hydrazine vapors are extremely toxic and the liquid is corrosive to skin. Methyl hydrazine is the strongest convulsant and the most toxic of methyl-substituted hydrazine derivatives. It is more toxic than hydrazine. At high doses, it is a strong central nervous system poison that can lead to convulsions and death. Skin rash may be aggravated by skin exposure.
화재위험
Extremely flammable; ignites spontaneously under almost all normal temperature conditions. Water used to extinguish a fire may cause pollution and should be diked for later disposal. Water may be ineffective in extinguishing fires due to the chemical's low flash point. Because of the wide flammability limits, low flash point, and reignition hazard, dry chemicals, carbon dioxide, water spray, and foam may not be as effective as water dilution of fire area. The vapor is heavier than air; thus Methylhydrazine may accumulate sufficiently to flash back. Methylhydrazine fires produce irritating nitrogen oxides. Ignites spontaneously in air when in contact with porous materials (e.g., earth, asbestos, wood, or cloth). Also ignites spontaneously on contact with strong oxidizing agents (e.g., fluorine, chlorine trifluoride, fuming nitric acid, and nitrogen tetroxide). Heat or flame should be avoided because chemical is extremely flammable and explosive.
Safety Profile
Suspected carcinogen
with experimental carcinogenic,
neoplastigenic, tumorigenic, and teratogenic
data. Poison by inhalation, ingestion, skin
contact, intraperitoneal, subcutaneous, and
intravenous routes. Experimental
reproductive effects. Human mutation data
reported. Corrosive to skin, eyes and mucous membranes. May self-ignite in air.
Very dangerous fire hazard when exposed
to heat or flame. To fight fire, use alcohol
foam, CO2, dry chemical. Explosive in the
form of vapor when exposed to heat or
flame. A powerful reducing agent. It is
hypergolic with many oxidants (e.g.,
dinitrogen tetraoxide and hydrogen
peroxide). When heated to decomposition it
emits toxic fumes of NOx.
잠재적 노출
MMH has been used as the propellant
in liquid propellant rockets; it is also used as a solvent and
as an organic intermediate.
Carcinogenicity
The carcinogenicity of methylhydrazine
has been extensively investigated. In two studies,
no compound-related increase in tumor incidence was
observed in mice treated orally with methylhydrazine
. In other studies, methylhydrazine produced
lung tumors in mice and malignant histiocytoma of the liver
and cecal tumors in hamsters when administered in drinking
water at concentrations of 0.01%. Potential carcinogenicity
from vapor exposure to methylhydrazine was also
investigated in rats, dogs, hamsters, and mice. Exposures to
methylhydrazine at concentrations of 0.02 ppm (rats and
mice only) and 0.2, 2, and 5 ppm (rats and hamsters only)were conducted for 6 h/day, 5 days/week, for a year, followed
by observation for 1 year.
환경귀착
Biological. It was suggested that the rapid disappearance of methylhydrazine in sterile and
nonsterile soil (Arrendondo fine sand) under aerobic conditions was due to chemical oxidation.
Although the oxidation product was not identified, it biodegraded to carbon dioxide in the
nonsterile soil. The oxidation product did not degrade in the sterile soil (Ou and Street, 1988).
운송 방법
UN1244 Methylhydrazine, Hazard class: 6.1;
Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid,
8-Corrosive material, Inhalation Hazard Zone A
Purification Methods
Dry with BaO, then distil it in a vacuum. Store it under nitrogen. [Beilstein 4 IV 3322.]
비 호환성
May form explosive mixture with air.
Methyl hydrazine is a highly reactive reducing agent and a
medium strong base. May explode if heated. Violent reaction with strong oxidizers, such as fluorine, chlorine, combustibles, nitric acid; hydrogen peroxide. Incompatible with
acids, alcohols, glycols, isocyanates, phenols, cresols;
porous materials, such as earth, asbestos, wood and cloth.
Oxides of iron or copper, manganese, lead, copper or their
alloys can lead to fire and explosions. Attacks cork, some
plastics, coatings and rubber.
폐기물 처리
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations
governing storage, transportation, treatment, and waste
disposal. There are 2 alternatives: Dilute with water,
neutralize with sulfuric acid, then flush to sewer with
large volumes of water or incinerate with added flammable solvent in furnace equipped with afterburner and
alkaline scrubber.
메틸히드리진 준비 용품 및 원자재
원자재
준비 용품
1-METHYL-3-PHENYL-1H-PYRAZOLE-5-CARBALDEHYDE
ETHYL 1-METHYL-5-PROPYL-1H-PYRAZOLE-3-CARBOXYLATE
(5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOL-4-YL)METHANAMINE
1-Methyl-5-propyl-1H-pyrazole-3-carboxylic acid amide ,97%
1-METHYL-4-NITRO-3-PROPYL-1H-PYRAZOLE-5-CARBOXYLIC ACID
1-METHYL-5-(TRIFLUOROMETHYL)-1H-PYRAZOL-3-OL
1,5-DIMETHYL-1H-PYRAZOLE-3-CARBOXYLIC ACID
5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOLE
1-Methyl-3-propyl-1H-pyrazole-5-carboxamide ,97%
5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOLE-4-CARBONYL CHLORIDE
2,2,2-TRIFLUORO-1-(3-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOL-4-YL)ETHANONE
4-CHLORO-1,3-DIMETHYLPYRAZOLO[3,4-B]PYRIDINE-5-CARBOXYLIC ACID
1,4,6-Trimethyl-1H-pyrazolo[3,4-b]pyridin-3-ylamine ,97%
1-METHYL-1H-PYRAZOLO[3,4-B]PYRIDIN-3-YLAMINE
1-METHYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE
ETHYL 2-METHYL-3-(TRIFLUOROMETHYL)PYRAZOLE-4-CARBOXYLATE
1-Methyl-3-propyl-1H-pyrazole-5-carbohydrazide ,97%
1,5-DIMETHYL-1H-PYRAZOLE-3-CARBONYL CHLORIDE
5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOL-4-AMINE
1,3-DIMETHYL-1H-THIENO[2,3-C]PYRAZOLE-5-CARBOXYLIC ACID
5-AMINO-3-METHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID ETHYL ESTER
3-ETHYL-1-METHYL-1H-PYRAZOLE-5-CARBOXYLIC ACID ETHYL ESTER
5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOLE-4-CARBONITRILE
1-METHYL-3-PROPYLPYRAZOLE-5-CARBOXYLIC ACID
프로카바진 수화염화물
1-METHYL-5-(1H-PYRROL-1-YL)-1H-PYRAZOLE-4-CARBOXYLIC ACID
5-Chloro-1,3-dimethyl-1H-pyrazole-4-carbaldehyde
ETHYL 1-METHYL-5-(1H-PYRROL-1-YL)-1H-PYRAZOLE-4-CARBOXYLATE
4-Bromo-1,3,5-trimethyl-1H-pyrazole
1,3,5-Trimethylpyrazole
1-METHYL-5-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLIC ACID
1-METHYL-3-PROPYL-1H-PYRAZOLE-5-CARBOXYLIC ACID ETHYL ESTER
(5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOL-4-YL)METHANOL
1,3-Dimethyl-5-hydroxypyrazole
안티피린
1-METHYL-5-PROPYL-1H-PYRAZOLE-3-CARBOXYLIC ACID
4-BROMO-1-METHYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE
5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOLE-4-CARBALDEHYDE
5-(TRIFLUOROMETHYL)-1-METHYL-1H-PYRAZOLE-4-CARBOXAMIDE
아짐설푸론