루틴
|
|
루틴 속성
- 녹는점
- 195 °C (dec.)(lit.)
- 알파
- D23 +13.82° (ethanol); D23 -39.43° (pyridine)
- 끓는 점
- 576.13°C (rough estimate)
- 밀도
- 1.3881 (rough estimate)
- 굴절률
- 1.7650 (estimate)
- 저장 조건
- Keep in dark place,Sealed in dry,2-8°C
- 용해도
- 피리딘: 50mg/mL
- 물리적 상태
- 가루
- 산도 계수 (pKa)
- 6.17±0.40(Predicted)
- 색상 색인 번호
- 75730
- 색상
- 노란색에서 녹색으로
- 수용성
- 12.5g/100mL
- Merck
- 8304
- 안정성
- 흡습성
- LogP
- -2.020 (est)
- CAS 데이터베이스
- 153-18-4(CAS DataBase Reference)
- EPA
- Rutin (153-18-4)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-36/37/38 | ||
| 안전지침서 | 24/25-36-26 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | VM2975000 | ||
| F 고인화성물질 | 8 | ||
| HS 번호 | 29381000 | ||
| 독성 | LD50 i.v. in mice: 950 mg/kg (propylene glycol soln) (Harrison) | ||
| 기존화학 물질 | KE-09600 |
| 그림문자(GHS): |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | ||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||||||||||||||||
| 예방조치문구: |
|
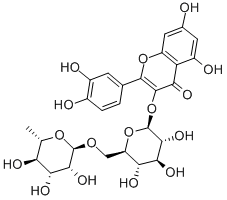
루틴 C화학적 특성, 용도, 생산
정의
이 품목은 콩과 회화나무(Sophora japonica L.)의 꽃, 꽃봉오리를 물 또는 에탄올로 추출하여 얻어지는 회화나무추출물, 마디풀과 메밀(Fagopyrum esculentum MOENCH.)의 전초를 물 또는 에탄올로 추출하여 얻어지는 메밀전초추출물, 콩과 팥(Phaseolus angularis CW. WIGHT.)의 전초를 물 또는 에탄올로 추출하여 얻어지는 팥전초추출물이다. 주성분은 플라보노이드계의 루틴(rutin, C27H30O16 = 610.51)이다. 다만, 색가조정, 품질보존 등을 위하여 희석제, 안정제 및 용제 등을 첨가할 수 있다.
순도시험
(1) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(2) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
확인시험
(1) 이 품목에 에탄올 10mL를 가하여 녹이고 염화제이철용액(1→50) 1~2방울을 가할 때, 액은 녹색을 띤 갈색을 나타낸다.
(2) 이 품목에 에탄올 5mL를 가하여 가온하고 녹인 후 염산 2mL 및 마그네슘분말 0.05g을 가할 때, 액은 서서히 적색을 나타낸다.
(3) 이 품목에 에탄올 500mL를 가하여 녹인 액은 파장 255nm 부근 및 375nm 부근에 극대흡수부가 있다.
(4) (3)의 용액 5mL에 수산화나트륨시액을 가하여 중성으로 하고 펠링시액 3mL를 가하여 수욕상에서 10분간 가열할 때, 적색의 침전이 생긴다.
개요
Rutin is widely found in nature and is almost contained in all of the Rutaceae and Sectaceae plants, especially abundant in Rutaceae, Rutaceae, Epacridaceae of leguminous, buckwheat of Polygonaceae, Eulali of Hypericum, Berchemia polyphylla var. leioclada of Tetranychidae, and wild wutong leaves of thistle, which are also used as raw materials in rutin extraction. In addition, it also exists in the Ilex pubescens of Aquifoliaceae, Forsythia of Oleaceae, pagoda tree pod of Leguminosae, tobacco, jujube, apricots, flavedo, tomatoes, and other plants.At present, rutin in China mainly extracted from the Sophora japonica Linn of leguminous, which is listed in top grade of Shen Nong’s Classic Materia Medica. In addition, tartary buckwheat, which is rich in rutin and flavonoids, is native to India and now produced in China’s northwest, southwest, north, south, and other places.
화학적 성질
Pale-Yellow Crystalline Solid물리적 성질
Appearance: light yellow or yellow-green needle crystal or crystalline powder, tastes slightly bitter, usually contains three crystal water, melting point at 176– 178 °C. Solubility: Rutin is soluble in methanol, pyridine, alkaline solution, and boiling water and hardly soluble in cold water, chloroform, carbon disulfide, ether, benzene, and petroleum ether.역사
In the mid-1930s, Hungarian scientist Szent Gyorgy firstly separated the flavonoid mixture. After the German pharmacy firstly made it into ranosine in 1942, the concept of vitamin P has been established worldwide. Further study proved that rutin was the most important flavonoids of vitamin P. These compounds were certified to have effects on many diseases in medical.Recently, the research of rutin mainly focuses on the extraction process improvement, pharmacological effects, and pharmacodynamics research, aiming at improvement of its bioavailability through the development of different dosage forms. As for the extraction process, new extraction and purification methods have been developed since the original alkali extraction acid precipitation method. These methods greatly improve its extraction efficiency and reduce cost, including hot water precipitation, hot water extraction with macroporous resin adsorbing purification, ultrasonic radiation, hot water extraction with alcohol precipitation, cold alkali percolation extraction with acid precipitation, continuous reflux extraction, ethanol extraction, supercritical CO2 extraction, and enzymatic hydrolysis .
In recent years, advanced rutin dosage forms, such as rutin cyclodextrin saturation, HPMC controlled release tablets, solid dispersion tablets, coprecipitate, and rutin effervescent particles, greatly improve the rutin dissolution rate and its bioavailability.
용도
Found in many plants, especially the buckwheat plant. Identity with Ilixanthin. Capillary protectant. Rutin is colored brown by tobacco enzyme under experimental conditions.정의
ChEBI: A rutinoside that is quercetin with the hydroxy group at position C-3 substituted with glucose and rhamnose sugar groups.Pharmacology
As a flavonoid substance, rutin has a significant protective effect on the cardiovascular system, including the endothelium-dependent vasodilation through NO-guanylate cyclase pathway, antagonization on platelet-activating factor (PAF), inhibition on subsequent reactions induced by PAF binding to its specific membrane receptor, and protection of myocardial cells .Rutin also has good free radical scavenging effects. Studies showed that rutin and its derivatives had a strong free radical scavenging effect, of which rutin possessed the strongest antioxidant activity. Rutin removed superoxide anion and hydroxyl radicals, exerted a strong anti-lipid peroxidation, protected mitochondria, and enhanced the activity of superoxide dismutase (SOD).
Clinical Use
Rutin is mainly used for the adjuvant treatment of hypertension and treatment for the prevention of other bleedings due to lack of rutin, such as cerebral hemorrhage, retinal hemorrhage, purpura, acute hemorrhagic nephritis, chronic bronchitis, and abnormal blood osmolality, restoration of capillary elastic embolism, and also for the prevention and treatment of diabetes and hyperlipidemia . Troxerutin, the most important active ingredient in hydroxy rutin, is used in the treatment of varicose veins/venous disorders, hemorrhoids, lymphedema, and postoperative edema, treatment of thrombosis and cerebrovascular disease, and also in the treatment of diabetes and liver disease. Since rutin has a mild effect with low cost and less adverse reactions, especially its remarkable effect on acute cerebral infarction, it is of great worth on promotion and application of rutin .부작용
Rutin is usually well tolerated. And since rutin is mostly derived from fruits and vegetables, it has mostly no adverse effects when taken orally, and rutin supplements may be safe when taken in doses of up to 600 mg per day for up to 12 weeks. Side effects may include headache or stomach upset.Purification Methods
The vitamin crystallises from MeOH or water/EtOH, dry it in air, then dry it further for several hours at 110o or in high vacuum at 120o. It forms yellow crystals from EtOH/Me2CO/H2O (2:1:1). It has also been purified by passing (0.5g) through a Kieselgel column (30 x 5cm) with EtOAc/MeOH/H2O (100:20:15), and after 750mL have passed through, the yellow fraction of 250mL gives the glycoside (0.3g) on evaporation. [H.rhammer et al. Chem Ber 101 1183 1968, Marini-Bettòlo Gazz Chim Ital 80 631 1950, Beilstein 18/5 V 519.]루틴 준비 용품 및 원자재
원자재
준비 용품
루틴 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Pharmyao, All for life--- A Specific Trading Platform For Reference Standard Material | +86-020-81716320 +8613602409664 |
sales@pharmyao.com | China | 173 | 58 |
| Chengdu ChenLv Herb Co.,Ltd | +undefined13608205856 |
maryextract@126.com | China | 127 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12457 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |
| Hebei Anlijie Biotechnology Co., Ltd | +8619031013551 |
ably@aljbio.com | China | 177 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |