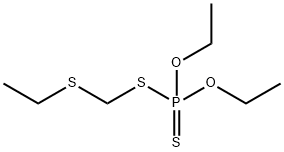
포레이트 C화학적 특성, 용도, 생산
개요
Phorate is a colorless
oil. The water solubility is 50 mg/L (25 ?C). It is miscible with
common organic solvents. Log Kow = 3.92. Phorate is
relatively unstable to hydrolysis in aqueous media; DT
50
values at pH 7 and 9 are 3.2 and 3.9 d, respectively.
Phorate is effective against sucking plant pests as a
systemic insecticide-acaricide and also has good contact
and vapor actions. It is usually formulated as granules.
The acute oral LD
50 for rats is 1.6–3.7 mg/kg. Inhalation
LC50 (1 h) for rats is 0.06–0.011 mg/L air. ADI is 0.5 μg/kg b.w.
화학적 성질
Phorate is a clear mobile liquid with a skunk-like odor
용도
Phorate is a non-biocumulative organophosphate used as an insecticide and acaricide. Phorate is an inhibitor of acetylcholinesterase and pseudocholinesterase.
일반 설명
Clear liquid with an objectionable odor. Used as an insecticide and acaricide; Phorate is applied to plants and soil.
공기와 물의 반응
Phorate is incompatible with the following: Water, alkalis [Note: Hydrolyzed in the presence of moisture and by alkalis.] .
반응 프로필
Organothiophosphates, such as Phorate, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
건강위험
Phorate is one of the more toxic organophosphorus insecticides. It is a cholinesterase inhibitor that acts on the nervous system, and produces toxicity similar to Parathion. The probable oral lethal dose for humans is less than 5 mg/kg, i.e. a taste (less than 7 drops) for a 70 kg (150 lb.) person.
화재위험
Shock can shatter containers, releasing the contents. When heated to decomposition, toxic fumes of sulfur oxides, phosphorus oxides, and nitrogen oxides are emitted. Hydrolyzed in water and alkalies.
농업용
Insecticide, Acaricide, Nematicide: Phorate is an organophosphorus insecticide and
acaricide used to control a wide variety of sucking and
chewing insects, leafhoppers, leafminers, mites, somenematodes, and rootworms. It is used on many crops, including
root and field crops such as corn, cotton, coffee,
potatoes, sugar beets, beans, peanuts, wheat, some ornamental
and herbaceous plants, and bulb. In the U.S., 80%
of the annual use of phorate is applied to corn, potatoes
and cotton. It is available in granular and emulsifiable
concentrate formulations. Phorate has been shown to be
responsible for a large number of bird kills and it is extremely
toxic to mammals. Not approved for use in EU
countries. A U.S. EPA restricted Use Pesticide (RUP).
U.S. Maximum Allowable Residue Levels for Phorate (40
CFR 180.206): bean 0.1 ppm; beet, sugar, roots 0.3 ppm;
beet, sugar, tops 3 ppm; coffee, bean 0.02 ppm; corn, forage
0.5 ppm; corn, grain 0.1 ppm; corn, sweet, kernel plus
0.1 ppm; cob with husks removed ppm; cotton, undelinted
seed 0.05 ppm; hop 0.5 ppm; peanut 0.1 ppm; potato
0.5 ppm; sorghum, grain, grain 0.1 ppm; sorghum, grain,
stover 0.1 ppm; soybean 0.1 ppm; sugarcane, cane 0.1 ppm;
wheat, grain 0.05 ppm; wheat, hay 1.5 ppm; wheat, straw
0.05 ppm.
상품명
AASTAR®[C]; AC 3911®; AGRIMET®;
AMERICAN CYANAMID 3,911®; EL 3911®;
EXPERIMENTAL INSECTICIDE 3911®; GEOMET®;
GRAMTOX®; GRANUTOX®; L 11/6®; METAPHOR®;
PHORATE-10G®; PHORIL®; RAMPART®;
TERRACLOR®; TERRATHION GRANULES®;
THIMENOX®; THIMET®; THEMET®; UMET®;
VEGFRU®; VERGFRU FORATOX®
Safety Profile
Poison by ingestion and sh contact routes. Experimental reproductive effects. Mutation data reported. A cholinesterase irhbitor. When heated to decomposition it emits toxic fumes of POx and SOx. See also PARATHION
잠재적 노출
Those engaged in the manufacture, formulation and application of this systemic and contact insecticide and acaricide. It is also used as a soil insecticide.
Carcinogenicity
When dogs were given phorate
via capsules at doses of 0.005, 0.01, 0.05, or 0.25 mg/kg/day
for 1 year, slight body tremors, marginal inhibition of body
weight gain, and RBC and brain cholinesterase inhibition
occurred in males given 0.25 mg/kg/day .
No evidence of carcinogenicity occurred in rats given
diets that contained 0, 1, 3, or 6 ppm phorate (equal to about
0, 0.05, 0.15, or 0.3 mg/kg/day) for 2 years .
Erythrocyte and brain cholinesterase inhibition occurred at
exposures of 3 and 6 ppm. No evidence of carcinogenicity
or other adverse effects occurred in mice given diets that
contained 0, 1, 3, or 6 ppm phorate (equal to about 0, 0.15,
0.45, and 0.9 mg/kg/day) for 78 weeks, other than a slight
decrease in body weight gain in females that were fed
6 ppm .
신진 대사 경로
Phorate is metabolised by an analogous route to that of disulfoton. The
principal route of phorate metabolism in all media is activation via
oxidation of the thioether group to the sulfoxide (rapid) and sulfone
(slower). Thioether oxidation occurs preferentially to oxidative desulfuration
of the P=S group to the oxon, which is usually only present in
trace amounts, and there is good evidence that the sulfoxide and sulfone
oxons arise via phorate sulfoxide and sulfone rather than phorate oxon.
The more polar thiooxidised metabolites are translocated in plants and
are responsible for the compound’s systemic action. Of all phorate’s
metabolites, phorate oxon sulfone is the most active inhibitor of acetylcholinesterase
(Bowman and Casida, 1957). Degradative metabolism
occurs via oxidative dealkylation of the phosphorodithioate group or
hydrolysis of the oxon.
신진 대사
The metabolic routes of phorate are essentially the
same in plants, animals, and soils, involving the oxidation
of the sulfide group into the sulfoxide then sulfone,
and oxidative desulfuration to the corresponding
oxons, followed by hydrolysis to diethyl hydrogen phosphorodithioate,
phosphorothioate, and phosphate. Phorate
protects plants for a relatively long time because of the
persistency of the sulfoxide metabolite in plants and in
soils. DT
50 in soil is 2–14 d.
운송 방법
UN3018 Organophosphorus pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2783 Organophosphorus pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous material. UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
비 호환성
Water, alkalis. Hydrolyzed in the presence of moisture and by alkalis; may produce toxic oxides of phosphorus and sulfur. Strong oxidizers may cause release of toxic phosphorus oxides. Organophosphates, in the presence of strong reducing agents such as hydrides, may form highly toxic and flammable phosphine gas. Keep away from alkaline materials.
폐기물 처리
In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
포레이트 준비 용품 및 원자재
원자재
준비 용품