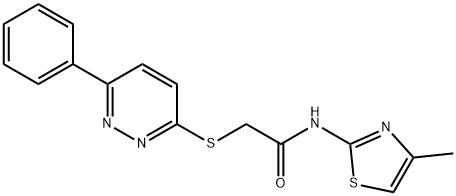
N-(4-Methyl-2-thiazolyl)-2-[(6-phenyl-3-pyridazinyl)thio]acetamide
|
|
N-(4-Methyl-2-thiazolyl)-2-[(6-phenyl-3-pyridazinyl)thio]acetamide 속성
- 녹는점
- 127 - 132°C
- 밀도
- 1.39±0.1 g/cm3(Predicted)
- 저장 조건
- 2-8°C
- 용해도
- DMSO: >20mg/mL
- 물리적 상태
- 가루
- 산도 계수 (pKa)
- 7.59±0.50(Predicted)
- 색상
- 페일베이지부터 베이지까지
안전
| WGK 독일 | 3 |
|---|
N-(4-Methyl-2-thiazolyl)-2-[(6-phenyl-3-pyridazinyl)thio]acetamide C화학적 특성, 용도, 생산
개요
VU0240551 is a K+/Cl- cotransporter 2 (KCC2) inhibitor (IC50 = 568 nM). It selectively inhibits KCC2 over Na+/K+/Cl- cotransporter 1 (NKCC1; IC50 = >50 μM) but does inhibit the activity of adenosine A1 and A3 receptors, as well as inhibits activity of L-type calcium channels at the benzothiazepine site and human ether-a-go-go-related gene (hERG) potassium channels by greater than 50% in a panel of 68 receptors, ion channels, and transporters at 10 μM. VU0240551 decreases potassium ion uptake by 70% in HEK293 cells expressing KCC2 when used at a concentration of 1 μM.용도
This molecule acts as an inhibitor of the K-Cl cotransporter KCC2, which is primarily responsible for maintaining intracellular Cl- concentrations including regulating an excess or lack of ions follow ing periods of under or overstimulation, as well as general dendritic development.Biochem/physiol Actions
VU0240551 is a potent, selective KCC2 inhibitor. KCC2 is a potassium-chloride exchanger expressed specifically in neurons. KCC2 functions to lower intracellular chloride concentrations below the electrochemical potential of the cells, thereby increasing the hyperexcitability of the neurons. KCC2 activity enhances GABA and other inhibitory neurotransmission and is implicated in pain processing. VU0240551 was discovered in a high-throughput screen, followed by directed medicinal chemistry. VU0240551 is selective for KCC2 over NKCC1. VU0240551 binds competitively to the K+ site and binds noncompetitively to the Cl- site. VU0240551 is the only small molecule with specificity for a KCC family member.N-(4-Methyl-2-thiazolyl)-2-[(6-phenyl-3-pyridazinyl)thio]acetamide 준비 용품 및 원자재
원자재
준비 용품
N-(4-Methyl-2-thiazolyl)-2-[(6-phenyl-3-pyridazinyl)thio]acetamide 공급 업체
글로벌( 55)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131981 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 |
jkinfo@jkchemical.com | China | 96815 | 76 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 |
3bsc@sina.com | China | 15848 | 69 |
| Haoyuan Chemexpress Co., Ltd. | 021-58950125 |
info@chemexpress.com | China | 7553 | 61 |
| MedChemexpress LLC | 021-58955995 |
sales@medchemexpress.cn | United States | 4863 | 58 |
| LETOPHARM LIMITED | +86-21-5821 5861 |
sales@letopharm.com | China | 2384 | 58 |
| Sigma-Aldrich | 021-61415566 800-8193336 |
orderCN@merckgroup.com | China | 51471 | 80 |
| SPIRO PHARMA | |
eric_feng1954@126.com | China | 9254 | 55 |
| EMMX Biotechnology LLC | 888-539-0666 |
info@emmx.com | United States | 8449 | 60 |
N-(4-Methyl-2-thiazolyl)-2-[(6-phenyl-3-pyridazinyl)thio]acetamide 관련 검색:
N-[1-(2-Fluorophenyl)-3-phenyl-1H-pyrazol-5-yl]-4-nitrobenzamide
Benzamide, 4-(cyclopropylmethoxy)-3-methoxy-N-[2-(1H-1,2,4-triazol-1-yl)-5-(trifluoromethoxy)phenyl]-
VU0134992(hydrochloride)
VU6005649
VU 591 hydrochloride
VU0810464
VU0483605
N-(4-Chloro-3-methoxyphenyl)-2-pyridinecarboxamide
1-(4-Methoxybenzyl)-5-trifluoromethoxyisatin
VU 6008667
(1R,2R)-N-([S]-1-{4-[5-broMo-2-oxo-2,3-dihydro-1H-benzo(d)iMidazol-1-yl]piperidin-1-yl}propan-2-yl)-2-phenylcyclopropanecarboxaMide
5-Amino-3,4-dimethyl-thieno[2,3-c]pyridazine-6-carboxylic acid 4-trifluoromethanesulfonyl-benzylamide
3-methylsulfanyl-7,8-dihydro-6H-cyclopenta[4,5]thieno[1,2-c]pyrimidin-1-amine
VU 0360172
VU 0365114
VU 0357121
VU 0360223
VU 0364439