Roxadustat C화학적 특성, 용도, 생산
개요
Roxadustat is an orally administered, small molecule hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor that is being developed by FibroGen, in collaboration with Astellas and AstraZeneca, for the treatment of anaemia in patients with dialysis-dependent chronic kidney disease (CKD), non-dialysis-dependent CKD and in patients with myelodysplastic syndromes.
용도
Roxadustat is a hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor used to increase white blood cell levels in blood and hematopoietic progenitor cells in bone marrow.
정의
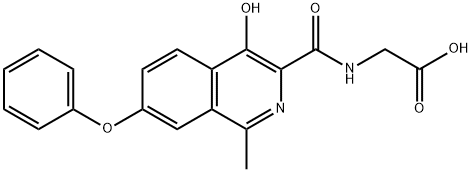
ChEBI: Roxadustat is an N-acylglycine resulting from the formal condensation of the amino group of glycine with the carboxy group of 4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxylic acid. It is an inhibitor of hypoxia inducible factor prolyl hydroxylase (HIF-PH). It has a role as an EC 1.14.11.2 (procollagen-proline dioxygenase) inhibitor and an EC 1.14.11.29 (hypoxia-inducible factor-proline dioxygenase) inhibitor. It is a member of isoquinolines, an aromatic ether and a N-acylglycine.
Synthesis
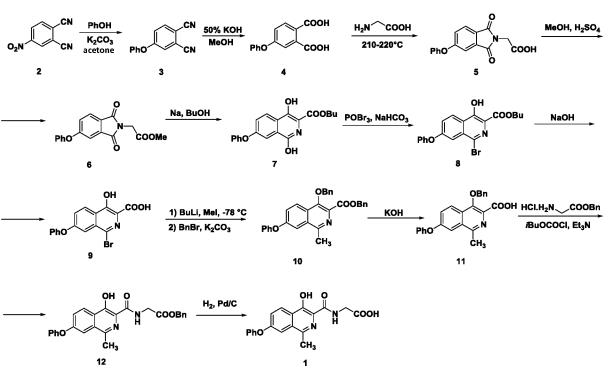
Preparation of roxadustat of formula 1 was described in a patent application WO2004108681, where the roxadustat is prepared in 11 steps from initial chemical compound 4-nitro-1,2-dicarbonitrile of formula 2. Reaction of dicarbonitrile of formula 2 with phenol and potash in the first step leads to preparation of corresponding phenoxy derivate of formula 3, 4-phenoxyphthalic acid of formula 4 is prepared by the following hydrolysis effect of potassium hydroxide in methanol, from which a solid-phase mixture with glycine is created for reaction in a melt with temperature of 210°C to 220°C, which provides corresponding phthalimide of formula 5. After that, phthalimide of formula 5 is esterified to corresponding methyl ester of formula 6. Then, expansion of the ring is performed by the effect of sodium butanolate to butyl-7-phenoxy-1,4-dihydroxy isoquinoline-3-carboxylate of formula 7. Hydroxyl group of derivative of formula 7 in a position 1 is then replaced by bromine using phosphorus oxybromide under the alkaline conditions resulting in creation of isoquinoline derivative of formula 8. Further the alkaline hydrolysis is carried out to provide an acid of formula 9. Then the lithiation is carried out, capture of lithium salt with methyl iodide, and in the second step, the hydroxyl group and carboxyl function are protected by benzyl group resulting in the derivative of formula 10. By the effect of potassium hydroxide the derivative of formula 10 is hydrolyzed to the carboxylic acid of formula 11, from which the derivative 12 is prepared under the conditions of glycine benzyl ester. The final step of synthesis of roxadustat of formula 1 is deprotection of benzyl groups by means of hydrogenation reaction (Diagram 1).
 A Scalable Synthesis of Roxadustat (FG-4592)
A Scalable Synthesis of Roxadustat (FG-4592)
IC 50
IC50 = 591.4 nM (HIF-PH).IC50 values for inhibition of peak and late components of delayed rectifier calcium currents are 5.71 and 1.32 μM in pituitary tumor cells.
신진 대사
Roxadustat is metabolized by cytochrome P450 (CYP)2C8 and uridine diphosphate-glucuronosyltransferase (UGT)1A9. Roxadustat is primarily metabolized through 2 main metabolic pathways: hydroxylation/oxidation followed by sulfation, producing metabolites 4'-hydroxy roxadustat and 4'-O-sulfate conjugates of 4'-hydroxy roxadustat, together accounting for 20% of the radioactive dose. The O-glucuronidation produces metabolite 4-O-β-glucuronide of roxadustat, representing 28% of the dose. Minor metabolic routes included glucosidation producing 4-O-β-glucoside of roxadustat (8.1% of the dose), acyl glucuronidation producing acyl-1-O-β-glucuronide of roxadustat (0.6% of the dose), and demethylation producing N-descarboxymethyl roxadustat oxide and Ndescarboxymethyl roxadustat (together ~3.6% of the dose).
Mode of action
Roxadustat is a novel, orally bioavailable, potent and reversible HIF-PH inhibitor (HIF-PHI) that transiently induces HIF stabilization and leads to a functional HIF transcriptional response that mimics the erythropoietic response associated with exposure of humans to intermittent hypoxia. roxadustat increases hemoglobin levels with a mechanism of action that is different from that of ESAs. Roxadustat activates the body’s natural protective response to reduced oxygen levels in the blood. This response involves the regulation of multiple, complementary processes that promote a coordinated erythropoietic response and increase the blood’s oxygen-carrying capacity.
Roxadustat 준비 용품 및 원자재
원자재
준비 용품