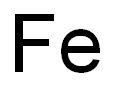철분
|
|
철분 속성
- 녹는점
- 1535 °C(lit.)
- 끓는 점
- 2750 °C(lit.)
- 밀도
- 7.86 g/mL at 25 °C(lit.)
- 인화점
- >230 °F
- 저장 조건
- 2-8°C
- 용해도
- H2O: 용해성
- 물리적 상태
- 철사
- 색상
- 은의
- Specific Gravity
- 7.86
- 냄새
- 냄새 없는
- 비저항
- 9.71 μΩ-cm
- 수용성
- 불용성
- 감도
- Moisture Sensitive
- Merck
- 13,5109
- 노출 한도
- ACGIH: TWA 2 mg/m3
OSHA: TWA 15 mg/m3; TWA 5 mg/m3
NIOSH: IDLH 1250 mg/m3; TWA 2.5 mg/m3
- 안정성
- 안정적인. 습한 공기 및 물과 천천히 반응합니다. 분진은 공기와 혼합되어 폭발성 또는 가연성 혼합물을 형성할 수 있습니다. 유기산, 강산화제, 물, 무기산과 호환되지 않습니다.
- CAS 데이터베이스
- 7439-89-6(CAS DataBase Reference)
- NIST
- Iron(7439-89-6)
- EPA
- Iron (7439-89-6)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/38-11-17 | ||
| 안전지침서 | 26-16-33-24/25 | ||
| 유엔번호(UN No.) | UN 3264 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | NO4565500 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 72052900 | ||
| 유해 물질 데이터 | 7439-89-6(Hazardous Substances Data) | ||
| 독성 | LD50 oral in rat: 30gm/kg | ||
| 기존화학 물질 | KE-21059 |
철분 C화학적 특성, 용도, 생산
순도시험
(1) 산불용물 : 이 품목 1g을 25mL의 묽은황산에 녹이고 수소가 발생하지 않을 때까지 가열한 후 여과하고 잔류물이 황산염반응이 없어질 때까지 물로 씻고 105℃에서 항량이 될 때까지 건조할 때, 그 양은 12.5mg 이하이어야 한다.
(2) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 8.0ppm 이하이어야 한다.
(3) 납 : 이 품목 1.0g을 달아 50mL의 비이커에 넣고 염산 8mL와 질산 2mL를 가하여 녹인 다음 수욕상에서 증발건고한 후 9N 염산 10mL를 가하여 녹인다. 필요시에 가온하여 녹인다. 이 액을 50mL 플라스크에 옮기고 물 10mL로 세척하여 그 세액을 합친 다음, 아스코브산-요오드화나트륨용액 20mL 및 트리옥틸포스핀옥시드용액 5mL를 넣고 30초 동안 흔들어 섞고 방치하여 층을 분리한다. 다시 물을 가하여 유기층을 플라스크의 목부분에 오도록 하고 흔들어 섞은 다음 정치하여 층을 분리한 후 유기용매 층을 시험용액으로 한다. 따로 납표준용액 1mL를 정확히 취하여 50mL 플라스크에 넣고 시험용액과 동일한 방법으로 조작하여 대조액으로 한다. 시험용액 및 대조액을 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 시험용액의 흡광도(발광강도)는 대조액의 흡광도(발광강도) 보다 커서는 아니 된다(10.0ppm 이하).
아스코브산-요오드화나트륨용액 : 아스코브산 10g 및 요오드화나트륨 19.3g을 물에 녹여 100mL로 한다.
트리옥틸포스핀옥시드용액 : 트리옥틸포스핀옥시드 5g을 메틸이소부틸케톤에 녹여 100mL로 한다.
(4) 수은 : 이 품목 1g을 묽은황산 30mL에 녹이고 과망간산칼륨용액(3→50) 1mL를 가한 다음, 시험용액 중의 과망간산칼륨의 자색이 없어지고 이산화망간의 침전이 없어질 때까지 염산히드록실아민용액(1→5)을 가한 후, 물을 가하여 100mL로 한 액을 시험용액으로 하여, 수은시험법 중 환원기화원자흡광광도법에 따라 시험할 때, 그 양은 5.0ppm 이하이어야 한다.
확인시험
이 품목에 묽은황산을 가하여 녹인 액은 확인시험법 중 제일철염의 반응을 나타낸다.
정량법
이 품목 약 0.2g을 정밀히 달아 300mL 삼각플라스크에 넣고 묽은황산 50mL를 가하여 분젠밸브마개를 하고 수욕상에서 가열하여 철을 완전히 녹인다. 식힌 다음 새로 끓여 식힌 물 50mL를 넣어 o-페난트로린시액 2방울을 넣고 0.1N 황산제이세륨용액으로 엷은 청색이 나타낼 때까지 적정한다. 따로 같은 방법으로 공시험을 한다.
0.1N 황산제이세륨용액 1mL = 5.585mg Fe
개요
Carbonyl iron is elemental iron produced by the decomposition of iron pentacarbonyl as a dark gray powder. When viewed under a microscope having a magnifying power of 500 diameters or greater, it appears as spheres built up with concentric shells. It is stable in dry air.화학적 성질
Silver-white malleable metal. The only metal that can be tempered. Mechanical properties are altered by impurities, especially carbon.Iron is highly reactive chemically, a strong reducing agent, oxidizes readily in moist air, reacts with steam when hot, t물리적 성질
Pure iron is a silvery-white, hard, but malleable and ductile metal that can be worked andforged into many different shapes, such as rods, wires, sheets, ingots, pipes, framing, and soon. Pure iron is reactive and forms many compounds with other elements. It is an excellentreducing agent. It oxidizes (rusts) in water and moist air and is highly reactive with most acids,releasing hydrogen from the acid. One of its main properties is that it can be magnetized andretain a magnetic field.The iron with a valence of 2 is referred to as “ferrous” in compounds (e.g., ferrous chloride= FeCl2). When the valence is 3, it is called “ferric” (e.g., ferric chloride = FeCl3).Iron’s melting point is 1,535°C, its boiling point is 2,750°C, and its density is 7.873g/cm3.Isotopes
There are 30 isotopes of iron ranging from Fe-45 to Fe-72. The following arethe four stable isotopes with the percentage of their contribution to the element’s naturalexistence on Earth: Fe-54 = 5.845%, Fe-56 = 91.72%, Fe-57 = 2.2%, and Fe-58 =0.28%. It might be noted that Fe-54 is radioactive but is considered stable because ithas such a long half-life (3.1×10+22 years). The other isotopes are radioactive and areproduced artificially. Their half-lives range from 150 nanoseconds to 1×105 years.Origin of Name
The name “iron” or “iren” is Anglo-Saxon, and the symbol for iron (Fe) is from ferrum, the Latin word for iron.Characteristics
Iron is the only metal that can be tempered (hardened by heating, then quenching in wateror oil). Iron can become too hard and develop stresses and fractures. This can be corrected byannealing, a process that heats the iron again and then holds it at that temperature until thestresses are eliminated. Iron is a good conductor of electricity and heat. It is easily magnetized,but its magnetic properties are lost at high temperatures. Iron has four allotropic states. Thealpha form exists at room temperatures, while the other three allotropic forms exist at varyinghigher temperatures.Iron is the most important construction metal. It can be alloyed with many other metals tomake a great variety of specialty products. Its most important alloy is steel.An interesting characteristic of iron is that it is the heaviest element that can be formed byfusion of hydrogen in the sun and similar stars. Hydrogen nuclei can be “squeezed” in the sunto form all the elements with atomic numbers below cobalt (27Co), which includes iron. Itrequires the excess fusion energy of supernovas (exploding stars) to form elements with protonnumbers greater than iron (26Fe).용도
Iron is a mineral used in food fortification that is necessary for the prevention of anemia, which reduces the hemoglobin concentra- tion and thus the amount of oxygen delivered to the tissues. sources include ferric ammonium sulfate, chloride, fructose, glycerophos- phate, nitrate, phosphate, pyrophosphate and ferrous ammonium sulfate, citrate, sulfate, and sodium iron edta. the ferric form (fe3+) is iron in the highest valence state and the ferrous form (fe2+) is iron in a lower valence state. the iron source should not discolor or add taste and should be stable. iron powders produce low discoloration and rancidity. it is used for fortification in flour, baked goods, pasta, and cereal products.생산 방법
Iron ore reserves are found worldwide. Areas with more than 1 billion metric tons of reserves include Australia, China, Brazil, Canada, the United States, Venezuela, South Africa, India, the former Soviet Union, Gabon, France, Spain, Sweden, and Algeria. The ore exists in varying grades, ranging from 20 to 70% iron content. North America has been fortunate in its ore deposits. There are commercially usable quantities in 22 U.S. states and in six Canadian provinces. In the United States the most abundant supplies, discovered in the early 1890s, are located in the Lake Superior region around the Mesabi Range. Other large deposits are found in Alabama, Utah, Texas, California, Pennsylvania, and New York. These deposits, particularly the Mesabi Range reserves, seemed inexhaustible in the 1930s when an average of 30 million tons of ore was produced annually from that one range. The tremendous demand for iron ore duringWorldWar II virtually tripled the output of the Mesabi Range and severely depleted its deposits of high-grade ore. The major domestic (U.S.) production is nowfrom crude iron ore, mainly taconite, a low-grade ore composed chiefly of hematite [FeO(OH) ·H2O] and silica found in the Great Lakes region.정의
iron: Symbol Fe. A silvery malleableand ductile metallic transition element;a.n. 26; r.a.m. 55.847; r.d.7.87; m.p. 1535°C; b.p. 2750°C. Themain sources are the ores haematite(Fe2O3), magnetite (Fe3O4), limonite(FeO(OH)nH2O), ilmenite (FeTiO3),siderite (FeCO3), and pyrite (FeS2).The metal is smelted in a blast furnaceto give impure pig iron, whichis further processed to give castiron, wrought iron, and varioustypes of steel. The pure element hasthree crystal forms: alpha-iron, stablebelow 906°C with a body-centredcubicstructure; gamma-iron, stablebetween 906°C and 1403°C with anonmagnetic face-centred-cubicstructure; and delta-iron, which isthe body-centred-cubic form above1403°C. Alpha-iron is ferromagneticup to its Curie point (768°C). The elementhas nine isotopes (mass numbers52–60), and is the fourth mostabundant in the earth’s crust. It is requiredas a trace element (see essentialelement) by living organisms.Iron is quite reactive, being oxidizedby moist air, displacing hydrogenfrom dilute acids, and combiningwith nonmetallic elements. It formsionic salts and numerous complexeswith the metal in the +2 or +3 oxidationstates. Iron(VI) also exists in theferrate ion FeO42-, and the elementalso forms complexes in which its oxidationnumber is zero (e.g. Fe(CO)5).일반 설명
A gray lustrous powder. Used in powder metallurgy and as a catalyst in chemical manufacture.공기와 물의 반응
Highly flammable. May react with water to give off hydrogen, a flammable gas. The heat from this reaction may ignite the hydrogen.반응 프로필
Iron is pyrophoric [Bretherick, 1979 p. 170-1]. A strong reducing agent and therefore incompatible with oxidizing agents. Burns in chlorine gas [Mellor 2, Supp. 1:380 1956]. Reacts with fluorine with incandescence [Mellor 13:314, 315, 1946-1947].위험도
Iron dust from most iron compounds is harmful if inhaled and toxic if ingested. Iron dustand powder (even filings) are flammable and can explode if exposed to an open flame. Asmentioned, excessive iron in the diet may cause liver damage.건강위험
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.화재위험
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.환경귀착
Iron occurs rarely by itself in nature due to the ease with which it forms compounds, especially in oxidation reactions. Many iron compounds are water soluble, leading to potentially high concentrations in water, especially in seawater. Iron is a necessary component of all life and it is therefore taken up readily by organisms from all sources.Purification Methods
Clean it in conc HCl, rinse in de-ionised water, then reagent grade acetone and dry it under vacuum.철분 준비 용품 및 원자재
원자재
준비 용품
철분 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 47465 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| Kindchem(Nanjing)Co.,Ltd | +86-025-025-85281586 +8618651653755 |
sales@kindchem.cn | China | 1229 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 |
ada@ipurechemical.com | China | 10326 | 58 |