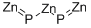인화아연 C화학적 특성, 용도, 생산
개요
Zinc phosphide, trizinc diphosphide, is an amorphous gray-black
powder with a garlic-like odor. It is practically insoluble
in water (decomposes slowly), ethanol, slightly soluble in
carbon disulfide, and benzene [9, p. 967].
Zinc phosphide is produced by heating finely powdered
zinc with phosphorus (10).
화학적 성질
Zinc phosphide is a gray crystalline solid.
용도
Zinc phosphide has been used since the second world war, and is most commonly used as an effective insecticide and rodenticide. Zinc phosphide is highly toxic due to its production of phosphine gas, and can cause symptoms such as nausea, vomiting and dyspnea in those (animal or human) that are exposed.
일반 설명
ZINC PHOSPHIDE is a dark gray granular solid. ZINC PHOSPHIDE is slowly decomposed by water giving off phosphine, a flammable poison gas. ZINC PHOSPHIDE is toxic by ingestion. ZINC PHOSPHIDE is used in medicine and as a rat poison.
반응 프로필
ZINC PHOSPHIDE is a reducing agent. They slowly generate flammable or noxious gases in contact with water. Phosphides react quickly upon contact with moisture or acids to give the very toxic gas phosphine; phosphides also can react vigorously with oxidizing materials. In general, materials in this group are incompatible with oxidizers such as atmospheric oxygen. They are violently incompatible with acids, particularly oxidizing acids.
건강위험
ZINC PHOSPHIDE is very caustic when ingested. ZINC PHOSPHIDE reacts with water and acid in the stomach and causes severe irritation. The probable oral lethal dose is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 70 kg (150 lb.) person. Most patients die after about 30 hours from peripheral vascular collapse secondary to the compound's direct effects. Extensive liver damage and kidney damage can also occur. Ingestion of 4-5 grams has produced death in human adults, but also doses of 25 to 50 grams have been survived. The lowest oral lethal dose reported for women is 80 mg/kg.
화재위험
When heated to decomposition, ZINC PHOSPHIDE emits toxic fumes of phosphorus and zinc oxides. Irritating oxides of phosphorus may be formed in fires. May ignite in presence of moisture. Contact with water produces flammable gas. Runoff to sewer may create fire or explosion hazard. Decomposed slowly by water giving off phosphine, a flammable poison gas. Reacts violently with concentrated sulfuric acid, nitric acid, and other oxidizing agents. Reacts with hydrochloric acid or sulfuric acid with the evolution of spontaneously flammable phosphine. May ignite in the presence of moisture, or evolve flammable gas. Stable unless exposed to moisture; toxic phosphine gas may then be released and collected in closed spaces. Hazardous polymerization may not occur.
농업용
Rodenticide: A U.S. EPA restricted Use Pesticide (RUP).
Registered for use in EU countries
. Zinc phosphide reacts with the acidic conditions in the gut to form phosphine
gas, which interferes with cell respiration. The rodenticide
may be used to control many species of rodents, including mice, ground squirrels, prairie dogs, voles, moles, rats,
muskrats, nutria and gophers. It may be used as an indoor
or outdoor spot treatment for rodents as well as around
burrows or underground in orchards, vineyards, various
food crops, range lands, and non-crop areas. Zinc phosphide is formulated as a bait/solid, dust, granular, pellet/
tablet or wettable powder and is also applied as a broadcast
treatment by ground or aerial applications.
상품명
BAKER BRAND®[C]; BLUE-OX®; E-Z
FLO®[C]; GOPHA-RID®; HOPKINS®; KILRAT®;
MOLETOX II®; MOUS-CON®; MR. KILL RAT®;
MR RAT GUARD®; NOTT ZINC PHOSPHIDE 93®;
RATOL®; ROBAN II AG®[C]; RUMETAN®; ZINC-
TOX®; ZP®
Safety Profile
Human poison by
ingestion causing nausea, vomiting, death.
Flammable when exposed to heat or flame.
This material is stable while kept dry. In
moist air, it decomposes slowly. Reacts
violently with acids or acid fumes to emit
the hghly toxic and flammable phosphine.
Violent reaction with concentrated sulfuric
acid, nitric acid, and oxidzing materials.
Incompatible with HCl, H2SO4. When
heated to decomposition it emits toxic
fumes of POx and ZnO. Used as an acute
rodenticide. See also PHOSPHIDES and ZINC COMPOUNDS.
잠재적 노출
It is used as an acute single feeding
rodenticide.
운송 방법
UN1714 Zinc phosphide, Hazard Class: 4.3;
Labels: 4.3-Dangerous when wet material, 6.1-Poisono us
materials.
비 호환성
Dust may form explosive mixture with
air. Heat and contact with water causes decomposition,
producing toxic and flammable fumes of phosphorus,
zinc oxides; and toxic and flammable phosphine gas.
Reacts violently with strong acids, including nitric,
hydrochloric acid or sulfuric acid with the evolution of
spontaneously flammable phosphine gas. Incompatible
with oxidizers (chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.);
contact can cause fires or explosions. Keep away from
alkaline materials, strong bases. carbon dioxide, halogenated
agents.
폐기물 처리
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform to EPA regulations governing
storage, transportation, treatment, and waste disposal.
Vanadium pentoxide may be salvaged or disposed of in a
sanitary landfill.
인화아연 준비 용품 및 원자재
원자재
준비 용품