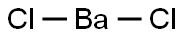바륨 클로라이드
|
|
바륨 클로라이드 속성
- 녹는점
- 963 °C (lit.)
- 끓는 점
- 1560°C
- 밀도
- 3.856 g/mL at 25 °C (lit.)
- 저장 조건
- 2-8°C
- 용해도
- H2O: 용해성
- 물리적 상태
- 염주
- 색상
- 하얀색
- Specific Gravity
- 3.9
- 수소이온지수(pH)
- 5-8 (50g/l, H2O, 20℃)
- 수용성
- 물과 메탄올에 용해됩니다. 산, 에탄올, 아세톤 및 에틸 아세테이트에 불용성입니다. 질산과 염산에 약간 용해됩니다.
- 감도
- Hygroscopic
- Merck
- 14,971
- 노출 한도
- ACGIH: TWA 0.5 mg/m3
NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3
- Dielectric constant
- 9.4(0.0℃)
- 안정성
- 안정적인.
- CAS 데이터베이스
- 10361-37-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,Xi,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-25-20-36/37/38-36/38-36 | ||
| 안전지침서 | 45-36-26-36/37/39 | ||
| 유엔번호(UN No.) | UN 3264 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | CQ8750000 | ||
| TSCA | Yes | ||
| HS 번호 | 2827 39 85 | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | III | ||
| 유해 물질 데이터 | 10361-37-2(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 118 mg/kg | ||
| 기존화학 물질 | KE-02037 |
바륨 클로라이드 C화학적 특성, 용도, 생산
개요
Barium dichloride is a white solid, odorless, hygroscopic chemical substance. Barium dichloride is used in the manufacture of pigments, in the manufacture of other barium salts and in fireworks to give a bright green color. It is one of the most common watersoluble salts of barium. Like other barium salts, it is toxic and imparts a yellow-green coloration to a flame. Barium chloride has wide application in the laboratory.화학적 성질
Barium chloride,BaCI2, is a colorless toxic salt with a melting point of 963°C. It is soluble in water. Barium chloride is used in metal surface treatment and as a rat poison.물리적 성질
Barium chloride has the formula, BaCl2 and is an ionic chemical compound. It is one of the most important water-soluble salts of barium-containing compounds. Like other barium salts, it is toxic and imparts a yellow-green coloration to a flame. It is also hygroscopic. Barium chloride was the by-product of the discovery of radium by Madame Curie (1898).When refining radium, the final separation resulted in barium chloride and radium chloride. BaCl2 crystallizes in both the cubic “fluorite” and “lead chloride” crystal structures, both of which accommodate the preference of the large Ba2+ ion for coordination numbers greater than six.제조 방법
Barium chloride can be prepared from barium hydroxide or barium carbonate, the latter being found naturally as the mineral “Witherite”. These basic salts react to give hydrated barium chloride. On an industrial scale, it is prepared via a two-step process from the mineral “Baryte”:BaSO4+4C→BaS+4CO (gas)
This first step requires high temperatures. The second step requires fusion of the reactants:
BaS+ CaCl2→BaCl2+CaS
The BaCl2 is then be leached out from the mixture with water. From water solutions of barium chloride, the dihydrate can then be crystallized as white crystals, BaCl2·2H2O, which are colorless, translucent rhomboidal tablets or lamellae. The dihydrate is stable in the air at room temperature, but loses one-half of its water above 55°C(131F), and becomes anhydrous at 121°C (250 F).
일반 설명
Any of a variety of substances that contain barium. Most are whitish colored crystalline solids. They tend to be soluble in water and denser than water. They may be toxic by inhalation or possibly skin absorption. They are often used to make other chemicals.공기와 물의 반응
Water soluble.반응 프로필
BARIUM CHLORIDE may react violently with BrF3 and 2-furan percarboxylic acid in its anhydrous form.위험도
Ingestion of 0.8 g may be fatal.화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.공업 용도
Barium chloride (BaCl2·2H2O) is a colorless, white powder highly soluble in water (25% at 10 °C). It is quite a toxic reagent. Barium chloride is used during borite flotation as an activator. Barium chloride also has a depressing effect on fluorite and cassiterite.Safety Profile
A poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Inhalation absorption of barium chloride equals 60-80%; oral absorption equals 10-30%. Experimental reproductive effects. Mutation data reported. See also BARIUM COMPOUNDS (soluble). When heated to decomposition it emits toxic fumes of Cl-.바륨 클로라이드 준비 용품 및 원자재
원자재
준비 용품
TARTRONIC ACID
수산화바륨
2,4,5,6-TETRAAMINOPYRIMIDINE
BARIUM NITRITE
(2,4-DIAMINOPTERIDIN-6-YL)METHANOL HYDROCHLORIDE HYDRATE
질산 바륨
바륨 카보네이트
수산화칼륨
몰리브데넘산 바륨(몰리브덴산 바륨)
아연 시아니드
염소산마그네슘
세노디올
크롬산바륨
디페닐아민 4-술폰산나트륨
나트륨
염화 아연
6-(Bromomethyl)-2,4-pteridinediamine hydrobromide
브로민산 칼륨
탄산, 리튬 염
브로민산나트륨
염화칼슘 2수화물
BARIUM PERCHLORATE TRIHYDRATE
과산화바륨
5-포밀살리실산
소다의 염산
수산화나트륨
산화크로뮴
barium fluxing agent
2,6-다이나이트로페놀
황산바륨
바륨 클로라이드 공급 업체
글로벌( 500)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 |
admin@zhanyaobio.com | China | 2136 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Dalian Richfortune Chemicals Co., Ltd | 0411-84820922 8613904096939 |
sales@richfortunechem.com | China | 303 | 57 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2967 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |