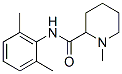
Mepivacaine
|
|
Mepivacaine 속성
- 녹는점
- 147-151°C
- 저장 조건
- -20°C Freezer
- 용해도
- 클로로포름(약간 용해됨), DMSO(약간 용해됨), 에탄올(약간 용해됨), 메탄올(약간 용해됨)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- pKa 7.73(H2O,t =25±0.2,I=0.01(NaCl)) (Uncertain)
- 색상
- 흰색에서 황백색까지
- InChI
- InChI=1S/C15H22N2O/c1-11-7-6-8-12(2)14(11)16-15(18)13-9-4-5-10-17(13)3/h6-8,13H,4-5,9-10H2,1-3H3,(H,16,18)
- InChIKey
- INWLQCZOYSRPNW-UHFFFAOYSA-N
- SMILES
- N1(C)CCCCC1C(NC1=C(C)C=CC=C1C)=O
- CAS 데이터베이스
- 22801-44-1(CAS DataBase Reference)
안전
Mepivacaine C화학적 특성, 용도, 생산
용도
Local anesthetic.일반 설명
Mepivacaine hydrochloride is available in 1% to 3% solutionsand is indicated for infiltration anesthesia, dental procedures,peripheral nerve block, or epidural block. The onset of anesthesiais rapid, ranging from about 3 to 20 minutes for sensoryblock. Mepivacaine is rapidly metabolized in the liver with50% of the administered dose excreted into the bile asmetabolites. The metabolites are reabsorbed in the intestineand excreted in the kidney with only a small percentage foundin the feces. Less than 5% to 10% of the administered dose isfound unchanged in the urine. The primary metabolic productsare the N-demethylated metabolite and the 3 and 4 phenolicmetabolites excreted as their glucuronide conjugates.Clinical Use
Mepivacaine hydrochloride [N-(2, 6-dimethylphenyl)-1-methyl 2-piperidinecarboxamide monohydrochloride] is an amino amide-type local anesthetic agent widely used to provide regional analgesia and anesthesia by local infiltration, peripheral nerve block, and epidural and caudal blocks. The pharmacological and toxicological profile of mepivacaine is quite similar to that of lidocaine, except that mepivacaine has a slightly longer duration of action and lacks the vasodilator activity of lidocaine. For this reason, it serves as an alternate choice for lidocaine when addition of epinephrine is not recommended in patients with hypertensive vascular disease.신진 대사
Mepivacaine undergoes extensive hepatic metabolism catalyzed by CYP1A2, with only a small percentage of the administered dosage (<10%) being excreted unchanged in the urine. The major metabolic biotransformations of mepivacaine are N-dealkylation (to give the N-demethylated compound 2′,6′-pipecoloxylidide) and aromatic hydroxylations. These metabolites are excreted as their corresponding glucuronides.Mepivacaine 준비 용품 및 원자재
원자재
준비 용품
Mepivacaine 공급 업체
글로벌( 115)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 |
admin@zhanyaobio.com | China | 2136 | 58 |
| Wuhan Haorong Biotechnology Co.,ltd | +8618565342920 |
sales@chembj.net | China | 269 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12453 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 |
admin@firsky-cn.com | China | 436 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 |
admin01@hsnm.com.cn | China | 2118 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Sigma Audley | +86-18336680971 +86-18126314766 |
nova@sh-teruiop.com | China | 524 | 58 |
| Shanghai Aosiris new Material Technology Co., LTD | +8615139564871 |
wrjmoon2000@163.com | China | 354 | 58 |
| Hubei XinRunde Chemical Co., Ltd. | +8615102730682 |
bruce@xrdchem.cn | CHINA | 566 | 55 |
| Wuhan Boyuan Import & Export Co., LTD | +8615175982296 |
Mike@whby-chem.com | China | 974 | 58 |
Mepivacaine 관련 검색:
N,N-다이메틸폼아마이드 노르말메틸포름아미드
Articaine
Bupivacaine
Levobupivacaine
Mepivacaine hydrochloride
Bupivacaine hydrochloride
(S)-Mepivacaine,L-(+)-Mepivacaine
Ropivacaine hydrochloride
Ropivacaine
Pipecolamide
Mepiquat
(R)-(+)-BUPIVACAINE HCL
(-)-Mepivacaine monohydrochloride,(R)-(-)-Mepivacaine monohydrochloride
1-BOC-PIPERIDINE-2-CARBOXYLIC ACID (2,6-DIMETHYL-PHENYL)-AMIDE
CARBOXAMIDE
MEPIVACAINE IMPURITY B
DL-Mepivacaine,Mepivacaine (base and/or unspecified salts),DL-1-METHYL-2'',6''-PIPECOLINOXYLIDIDE(MEPIVACAINE BASE)