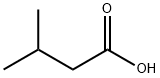
이소발레릴산 C화학적 특성, 용도, 생산
개요
Isovaleric acid has a characteristic disagreeable odor. It is extremely
penetrating and persistent with a sour taste. May be synthesized by
oxidation of isoamyl alcohol or isovaleric aldehyde.
화학적 성질
Isovaleric acid has a characteristic disagreeable, rancid, cheese-like odor. It is extremely penetrating and persistent with
a sour taste. May consist of one or a mixture of isomers or n-pentanoic acid and/or 2- or 3-methyl butanoic acid.
Consumption: Annual: 1850.00 lb
출처
Of the three possible isomers of n-valeric acid, only isovaleric acid has extensive application in flavoring;
originally reported in seal and dolphin fat; subsequently isolated from valerian. Also reported found in the essential oils of cypress,
citronella, laurel leaves, cajeput, Cymbopogon javanensis, hops, Persea pubescens, geranium, American peppermint, spearmint,
rosemary, lemongrass, Eucalyptus goniocalyx and other spp., tobacco, Monarda fistulos, Thymus mastichina, Artemisia frigida,
and probably in lavender; reported among the constituents of petitgrain lemon. Also reported found in many foods including apple,
currants, guava, grapes, papaya, peach, pineapple, raspberry, strawberry, potato, bell pepper, vinegar, breads, many cheeses, fish,
chicken, lamb, hop oil, beer, cognac, whiskies, cider, sherry, grape wines, rum, cocoa, tea, coffee, honey, soybean, passion fruit,
mushrooms, marjoram, plum, brandy, starfruit, trassi, rice, jackfruit, sake, sukiyaki, buckwheat, corn oil, cashew apple, malt, wort,
Bourbon vanilla, shrimp, mussels, cherimoya, Cape gooseberry and Chinese quince frui
용도
Isovaleric acid is used extensively as a flavoring ingredient in
nonalcoholic beverages and in foods such as ice cream,
candy, baked goods, and cheese, as a fragrance ingredient
in perfumes, and as a chemical intermediate in the
manufacture of sedatives and other pharmaceutical products.
It is also used as an extractant of mercaptans from petroleum
hydrocarbons, a vinyl stabilizer, and as an intermediate in the
manufacture of plasticizers and synthetic lubricants.
제조 방법
By oxidation of isoamyl alcohol or isovaleric aldehyde
정의
ChEBI: A C5, branched-chain saturated fatty acid.
일반 설명
Isovaleric acid is a colorless liquid with a penetrating odor. Isovaleric acid is slightly soluble in water. Isovaleric acid is corrosive to metals and to tissue.
공기와 물의 반응
Isovaleric acid is slightly soluble in water.
위험도
Strong irritant to tissue.
건강위험
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
이소발레릴산 준비 용품 및 원자재
원자재
준비 용품