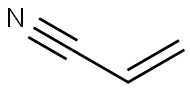
아크릴로나이트릴
|
|
아크릴로나이트릴 속성
- 녹는점
- -83 °C (lit.)
- 끓는 점
- 77 °C (lit.)
- 밀도
- 0.806 g/mL at 20 °C
- 증기 밀도
- 1.83 (vs air)
- 증기압
- 86 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.391(lit.)
- 인화점
- 32 °F
- 저장 조건
- 2-8°C
- 용해도
- 73g/L
- 물리적 상태
- 액체
- 색상
- 투명한
- 수소이온지수(pH)
- 6.0-7.5 (50g/l, H2O, 20℃)
- 냄새
- 2~22ppm에서 약한 피리딘 유사 냄새
- Odor Threshold
- 8.8ppm
- 폭발한계
- 2.8-28%(V)
- 수용성
- 녹는. 7.45g/100mL
- 감도
- Light Sensitive
- Merck
- 14,131
- BRN
- 605310
- Henry's Law Constant
- 1.30 at 30.00 °C (headspace-GC, Hovorka et al., 2002)
- 노출 한도
- NIOSH REL: TWA 1 ppm, 15-min C 1 ppm, IDLH 85 ppm; OSHA PEL: TWA 2 ppm, 15-min C 10 ppm; ACGIH TLV: TWA 2 ppm.
- Dielectric constant
- 33.009999999999998
- LogP
- 0.017 at 21℃
- CAS 데이터베이스
- 107-13-1(CAS DataBase Reference)
- IARC
- 2B (Vol. 71) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,T,N,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-11-23/24/25-37/38-41-43-51/53-39/23/24/25-62-63 | ||
| 안전지침서 | 53-9-16-45-61-36/37 | ||
| 유엔번호(UN No.) | UN 1093 3/PG 1 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | AT5250000 | ||
| F 고인화성물질 | 8 | ||
| 자연 발화 온도 | 481 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | I | ||
| HS 번호 | 29261000 | ||
| 유해 물질 데이터 | 107-13-1(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 0.093 g/kg (Smyth, Carpenter) | ||
| IDLA | 60 ppm | ||
| 기존화학 물질 | KE-29393 | ||
| 유해화학물질 필터링 | 97-1-170 | ||
| 중점관리물질 필터링 | 별표1-56 | ||
| 사고대비 물질 필터링 | 24 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 아크릴로니트릴 및 이를 0.1% 이상 함유한 혼합물 |
아크릴로나이트릴 C화학적 특성, 용도, 생산
용도
제품의 권고 용도와 사용상의 제한 본 제품은 실험실 및 연구용 시약 외의 용도로는 사용할 수 없음 다.안전성
가.눈에 들어갔을 때 많은 양의 물이나 생리식염수로 15분 이상 눈을 세척하고 즉시 의사의 치료를 받을 것.
나.피부에 접촉했을 때 오염된 의복 및 신발을 즉시 벗고 15분 이상 다량의 물과 비누로 씻
을 것.
다.흡입했을 때 노출로부터 환자를 즉시 신선한 공기가 있는 곳으로 옮기고 호흡정
지 및 곤란시 인공호흡 실시 및 의사의 치료를 받을 것.
라.먹었을 때 구토를 하지 않도록 하고 즉시 의사의 치료를 받을 것.
마.기타 의사의 주의사항 섭취의 경우 위 세척을 고려할 것.
흡입의 경우에는 산소의 공급을 고려할 것.
의료인력이 해당물질에 대해 인지하고 보호조치를 취하도록 할 것.
화학적 성질
Acrylonitrile is a colorless, flammable liquid. Its vapors may explode when exposed to an open flame. Acrylonitrile does not occur naturally. It is produced in very large amounts by several chemical industries in the United States and its requirement and demand has increased in recent years. The largest users of acrylonitrile are chemical industries that make acrylic and modacrylic fi bers, high impact acrylonitrile-butadiene-styrene (ABS) plastics. Acrylonitrile is also used in business machines, luggage, and construction material, in the manufacturing of styrene-acrylonitrile (SAN) plastics for automotive and household goods, and in packaging material. Adiponitrile is used to make nylon, dyes, drugs, and pesticides.용도
Acrylonitrile is used in the production of acrylic fibers, resins, and surface coating; as an intermediate in the production of pharmaceuticals and dyes; as a polymer modifier; and as a fumigant. It may occur in fire-effluent gases because of pyrolyses of polyacrylonitrile materials. Acrylonitrile was found to be released from the acrylonitrile–styrene copolymer and acrylonitrile–styrene–butadiene copolymer bottles when these bottles were filled with food-simulating solvents such as water, 4% acetic acid, 20% ethanol, and heptane and stored for 10 days to 5 months (Nakazawa et al. 1984). The release was greater with increasing temperature and was attributable to the residual acrylonitrile monomer in the polymeric materials.정의
ChEBI: Acrylonitrile is a nitrile that is hydrogen cyanide in which the hydrogen has been replaced by an ethenyl group. It is very toxic and irritant but is also a sensitizer. It caused both irritant and allergic contact dermatitis in a production manufacture.공기와 물의 반응
Highly flammable. Soluble in water.반응 프로필
ACRYLONITRILE produces poisonous hydrogen cyanide gas on contact with strong acids or when heated to decomposition. Reacts violently with strong oxidizing agents (dibenzoyl peroxide, di-tert-butylperoxide, bromine) [Sax, 9th ed., p. 61]. Rapidly ignites in air and forms explosive mixtures with air. Polymerizes violently in the presence of strong bases or acids. Underwent a runaway reaction culminating in an explosion on contact with a small amount of bromine or solid silver nitrate [Bretherick, 5th ed., 1995, p. 404].색상 색인 번호
Acrylonitrile is a raw material used extensively in industry, mainly for acrylic and modacrylic fibers, acrylonitrile-butadiene-styrene and styrene-acrylonitrile resins, adiponitrile used in nylon’s synthesis, for nitrile rubber, and plastics. It is also used as an insecticide. This very toxic and irritant substance is also a sensitizer and caused both irritant and allergic contact dermatitis in a production manufacturer.잠재적 노출
Acrylonitrile is used in the manufacture of synthetic fibers, polymers, acrylostyrene plastics, acrylonitrile butadiene styrene plastics, nitrile rubbers, chemicals, and adhesives. It is also used as a pesticide. In the past, this chemical was used as a room fumigant and pediculicide (an agent used to destroy lice).Carcinogenicity
Acrylonitrile is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.저장
Work with acrylonitrile should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Acrylonitrile should be used only in areas free of ignition sources. Containers of acrylonitrile should be stored in secondary containers in the dark in areas separate from oxidizers and bases.운송 방법
UN1093 Acrylonitrile, stabilized, Hazard Class 3; Labels: 3 Flammable liquids, 6.1-Poisonous materialsPurification Methods
Wash acrylonitrile with dilute H2SO4 or dilute H3PO4, then with dilute Na2CO3 and water. Dry it with Na2SO4, CaCl2 or (better) by shaking with molecular sieves. Fractionally distil it under N2. It can be stabilised by adding 10ppm tert-butyl catechol. Immediately before use, the stabilizer can be removed by passage through a column of activated alumina (or by washing with 1% NaOH solution if traces of water are permissible in the final material), followed by distillation. Alternatively, shake it with 10% (w/v) NaOH to extract inhibitor, and then wash it in turn with 10% H2SO4, 20% Na2CO3 and distilled water. Dry for 24hours over CaCl2 and fractionally distil under N2 taking fraction boiling at 75.0-75.5oC (at 734mm). Store it with 10ppm tert-butyl catechol. Acrylonitrile is distilled off when required. [Burton et al. J Chem Soc, Faraday Trans 1 75 1050 1979, Beilstein 2 IV 1473.]비 호환성
Acrylonitrile is reactive with, and must be kept away from, strong oxidizers, especially bromine. Use extreme care to keep Acrylonitrile away from strong bases, strong acids, copper, copper alloys, ammonia and amines. Contact with these chemicals can cause a chemical reaction resulting in a fire or explosion. Chemical compatibility should also be determined before Acrylonitrile comes in contact with any other chemical.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration with provision for nitrogen oxides removal from effluent gases by scrubbers or afterburners. A chemical disposal method has also been suggested involving treatment with alcoholic NaOH; the alcohol is evaporatedand calcium hypochlorite added; after 24 hours the product is flushed to the sewer with large volumes of water. Recovery of acrylonitrile from acrylonitrile process effluents is an alternative to disposal.아크릴로나이트릴 준비 용품 및 원자재
원자재
실리카겔
dilute sulphuric acid
락토니트릴
Magnesium carbonate
프로필렌
Extraction column
산소, 냉각된 액체
트라이메틸아민(트리메틸아민)
비스무트
시안화수소
폴리아크릴산
황산암모늄
준비 용품
전분글리콜산나트륨
3-PIPERAZIN-1-YL-PROPIONITRILE
Leather lustering agent
Fursultiamine
Cibenzoline
디에틸에틸렌디아민
N-(3-Aminopropyl)-imidazole
Chlorpyrifos,E.C.
N-(ETHOXYCARBONYL)-N-(ETHOXYCARBONYKLETHYL)GLYCINE ETHYL ESTER
N-(2-CYANOETHYL)PYRROLE
N-메틸올아크릴아마이드
3-CARBETHOXY-2-PIPERIDONE
디메틸아미노프로피온니트릴
디라우릴 3,3`-티오디프로피오네이트
클로로(3-)피오니트릴
Polyacrylamide dry powder,cationic
2-chloro-2-trichloroethyl-3,3-dimethyl cyclobutenone
modified acrylic resin emulsion J
rubber latex BA
acrylic resin emulsion FX-1
Nitrile rubber NBR
crosslinking aent DTF-3
N,N-Bis(cyanoethyl)aniline
coupling agent NBC-1
2,3-디브로모프로피오 니트릴
brubber latex 202BA
DIETHYL 2-(2-CYANOETHYL)MALONATE
플루디옥소닐
비스(아미노프로필)피페라진
Methyl 1-Methyl-2-oxopyrrolidine-3-carboxylate
N-(2-CYANOETHYL)GLYCINE
티오디글리콜 산
Binder for coating printing
2,3-Dichloropropionitrile
Polyacrylamide dry powder,non-ionic
다이에틸아미노트라이메틸렌아민
interpenetrating network ion exchange resin
TXG retention aid
1,2-디브로모-2,4-디시아노부탄
N,N-Dibenzylhydroxylamine
아크릴로나이트릴 공급 업체
글로벌( 440)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 |
info@longyupharma.com | China | 2531 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 47465 | 58 |