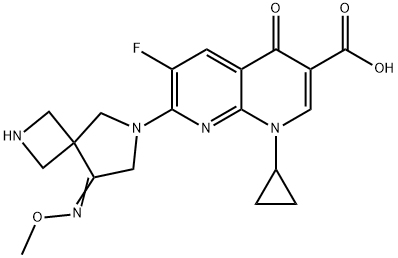
Zabofloxacin C화학적 특성, 용도, 생산
개요
Zabofloxacin
is a quinolone antibiotic originally developed by Dong
Wha Pharmaceuticals and licensed to Pacific Beach Biosciences
in 2007. In March 2015, Korea’s Ministry of Food and Drug
Safety (MFDS) approved zabofloxacin for the treatment of
acute bacterial exacerbation of chronic obstructive pulmonary
disease (ABE-COPD). In 2016, zabofloxacin gained approval
from the USFDA for the treatment of community-acquired
pneumonia. ABE-COPD is caused by respiratory tract and
pulmonary parenchyma that cause chronic pulmonary inflammation
and obstruction in the respiratory tract, which leads to
irreversible damage. In the nonclinical evaluation process,
zabofloxacin showed strong antibiotic activity on respiratory
germs (e.g., Streptococcus pneumonia, S. Haemophilus, S.
moraxella) and was the most potent antibacterial agent against
penicillin-resistant S. pneumoniae (PRSP) in the murine
systemic infection model.
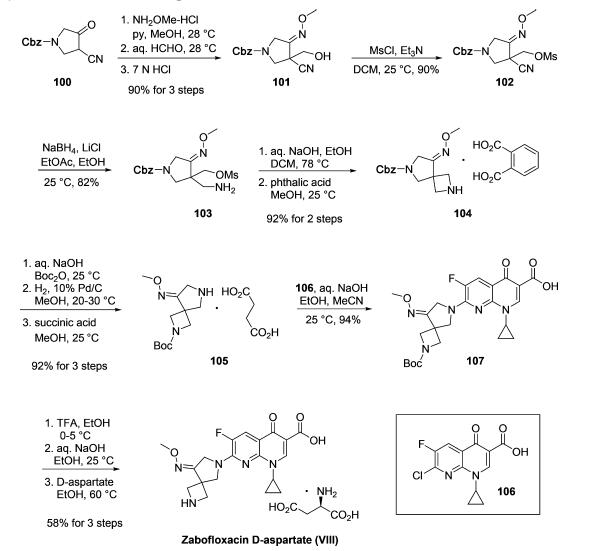
Synthesis
The synthesis of zabofloxacin leverages the wide commercial
availability of chloronaphthyridinone acid 106 to essentially
reduce the task to the construction of functionalized
diazaspirocyclic pyrrolidine 105. As described
in a series of patents from researchers at Dong Wha who have exemplified the synthesis on multikilogram scale, the route
began with first converting the commercially available ketone
100 to the corresponding oxime followed by formylation to
give oximyl alcohol 101. Next, mesylation of the alcohol was
followed by conversion of the nitrile to the corresponding
amine 103. An intramolecular ring closing step then occurred
to secure the azetidine using aqueous sodium hydroxide. Salt
formation with phthalic acid furnished 104 in good yield. Next,
Boc-protection of the azetidine followed by hydrogenative Cbz
removal and treatment with succinic acid resulted in the
formation of amine salt 105, and this was followed by a
substitution reaction with 106 to deliver the Boc-protected
zabofloxacin structure 107. Lastly, removal of Boc via TFA
followed by basification and subjection to D-aspartate in warm
ethanol furnished zabofloxacin D-aspartate (VIII) in 56% yield
for the three-step sequence.
Zabofloxacin 준비 용품 및 원자재
원자재
준비 용품