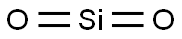해사
|
|
해사 속성
- 녹는점
- 1610 °C(lit.)
- 끓는 점
- 2230 °C
- 밀도
- 2.6 g/mL at 25 °C(lit.)
- 굴절률
- n
20/D 1.544(lit.)
- 저장 조건
- no restrictions.
- 용해도
- insoluble in H2O, acid solutions; soluble in HF
- 물리적 상태
- 가루
- 색상
- 하얀색
- Specific Gravity
- 2.2-2.6
- 수소이온지수(pH)
- 5-8 (400g/l, H2O, 20℃)(slurry)
- 수용성
- 불용성
- 노출 한도
- ACGIH: TWA 0.025 mg/m3
OSHA: TWA 50 μg/m3
NIOSH: IDLH 50 mg/m3; TWA 0.05 mg/m3
- Dielectric constant
- 4.2(0.0℃)
- 안정성
- 안정적인.
- InChIKey
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N
- CAS 데이터베이스
- 14808-60-7(CAS DataBase Reference)
- IARC
- 1 (Vol. Sup 7, 68, 100C) 2012
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38-48/20 | ||
| 안전지침서 | 26-24/25-22 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | ZG6800000 | ||
| TSCA | Yes | ||
| HS 번호 | 25061000 | ||
| 유해 물질 데이터 | 14808-60-7(Hazardous Substances Data) | ||
| 독성 | LDLo intravenous in dog: 20mg/kg | ||
| IDLA | 25 mg/m3 | ||
| 기존화학 물질 | KE-29983 | ||
| 중점관리물질 필터링 | 별표1-174 |
해사 C화학적 특성, 용도, 생산
용도
벌킹제, 불투명화제, 안티케이킹제, 연마제, 현탁화제(비계면활성), 흡수제용도
도자기의 주성분인 동시에 광 파이버(광섬유), 수정진동자등 하이테크의 기본이 되는 소재로서 중요하다.용도
석영 유리는 내열ㆍ내열충격ㆍ내약품성 등을 이용하여 이 화학실험 기구와 반도체 제작에 있어 필요 불가결하다. 또 석영 유리분과 규석분은 에폭시 수지에 섞어서 IC 등의 밀봉용에 사용된다. 합성수정은 수정 진동자로서 시계, 통신기, VTR 등에 사용되는 외에 일렉트로닉스 관련 광학부품으로도 많이 사용되고 있다.개요
Quartz is a colorless solid that exists in numerous crystalline forms. Oxygen and silicon are the two most common elements in the earth's crust, and Quartz is the principal component of sand. Quartz is used biologically, most notably by phytoplankton diatoms and the zooplankton radiolarians in their shells. Quartz, SiO2, should not be confused with Quartz or silicones. Quartz contain the basic tetrahedral unit SiO44-bonded to metal ions such as aluminum, iron, sodium, magnesium, calcium, and potassium to form numerous Quartz minerals. Silicones are synthetic polymers made of monomers with at least two silicon atoms combined with an organic group and generally containing oxygen.화학적 성질
granular abrasive solid of varied colour, depending upon other출처
Quartz is the oxide of the nonmetallic element silicon, is the commonest of minerals, and appears in a greater number of forms than any other. Its formula is SiO2. Quartz commonly occurs in prismatic hexagonal crystals terminated by a pyramid. This pyramid is due to the equal development of two rhombohedrons, and may be observed in cases where one rhombohedron predominates. Cleavage is not observed; the fracture is typically conchoidal; hardness is 7; specific gravity, 2.65; luster, vitreous to greasy or dull; colorless to white, pink, purple, yellow, blue, green, smoky brown to nearly black; transparent to opaque.Characteristics
Quartz can exist in either a crystalline or noncrystalline form. In Quartz, SiO2 exists in the natural crystalline state and possesses long-range order, with the silicon atom covalently bonded to oxygen atoms in a tetrahedral arrangement in a regular repeating pattern. Glass is an example of noncrystalline Quartz. Although natural glasses exist, Quartz glasses are produced when Quartz is heated to an elevated temperature and then rapidly cooled. The rapid cooling does not allow the SiO2 to form a regular crystalline structure with long-range order. The result is a solid that behaves like a viscous liquid when heated. Glass is sometimes called a solid solution and fl ows at a very slow rate. This can sometimes be seen in old window glass where the bottom is slightly thicker than the top. The actual structures form a three-dimensional tetrahedral pattern. Quartz is sold as sand and its main uses are for glass; ceramics; foundry sand, a source of silicon in the chemical industry; as a filtration media; a filler/extender; an abrasive; and as an adsorbent.용도
Sand, white silica has been employed as a solid sample to evaluate the pore-volume variations during fluid-rock interaction experiments.정의
A purple form of the mineral quartz (silicon(IV) oxide, SiO2) used as a semiprecious gemstone. The color comes from impurities such as oxides of iron.화학 반응
SiO2 shows strong absorption at 8.5 mm, 9.2 mm, and 12.5 mm (refer to the Silica) and SiO at 10.4 m. Thin films of SiO2 are fabricated by oxidizing the SiO in air after deposition of SiO by vacuum evaporation.일반 설명
The product is sand, white quartz (SiO2). Its reaction with alkaline NaNO3 solutions containing dissolved Al at 89°C has been investigated.위험도
Avoid inhalation of fine particles.건강위험
Exposure to Quartz can result in the disease called silicosis. Silicosis is a disabling, nonreversible, and sometimes fatal lung disease caused by overexposure to respirable crystalline Quartz. In silicosis, Quartz particles enter the lung where they become trapped, producing areas of swelling. The swelling results in nodules that become progressively larger as the condition worsens. Silicosis is defined at several levels of severity: chronic silicosis, accelerated silicosis, and acute silicosis. Chronic silicosis results from long-term (20 years) exposure to low concentrations of Quartz, whereas acute silicosis is the result of a short-term exposure (a year or less) to high concentrations. Symptoms may not be obvious in cases of chronic silicosis and x-ray screening is recommended for at-risk groups. These include sand-blasters, miners, laborers who regularly saw, drill, and jack-hammer concrete, and general construction such as tunnel drilling. In advanced stages of silicosis, individuals have difficulty breathing, especially when active.공업 용도
Crushed and graded quartz is used as the abrasive in flint sandpapers. Almost any deposit of massive white quartz is suitable. Being the cheapest of all the abrasive-coated paper, this product is still sold in fair amounts, mainly in hardware stores and by small jobbers. It is made only in the form of paper, not as cloth. True chalk flint from England and France is used extensively for this purpose in Europe; it has better cutting qualities and longer life than ordinary quartz. Crushed and ground sand, sandstone, powdered quartz, and silt are sometimes used in hand soaps, scouring compounds, and harsher metal polishes.Safety Profile
Confirmed carcinogen with experimental carcinogenic, tumorigenic, and neoplastigenic data. Experimental poison by intratracheal and intravenous routes. An inhalation hazard. Human systemic effects by inhalation: cough, dyspnea, liver effects. Incompatible with OF2, vinyl acetate. See also other silica entries잠재적 노출
Cristobalite is used in the manufacture of water glass, refractories, abrasives, ceramics and enamels. Quartz is used as a mineral, natural or synthetic fiber. Tridymite is used as a filtering and insulating media and as a refractory material for furnace linings. Workers are potentially exposed to crystalline silica in such industries as granite quarrying and cutting, foundry operations; metal, coal, dentistry, painting, and nonmetallic mining; and manufacture of clay and glass products.Carcinogenicity
Quartz was not mutagenic in bacterial assays; both positive and negative results have been reported in a wide variety of in vivo and in vitro genotoxic assays.비 호환성
Violent reactions with powerful oxidizers: fluorine, chlorine trifluoride; manganese trioxide; oxygen difluoride, hydrogen peroxide, etc.; acetylene; ammonia.폐기물 처리
Sanitary landfill해사 준비 용품 및 원자재
원자재
실리카겔
암모니아수
dilute sulphuric acid
AMBERSEP 900, OH FORM ION-EXCHANGE RESIN
Reaction tank
실리콘 분
규산소다
코발트 염화물, 헥사수화물
황산
준비 용품
수소화칼슘
오스뮴산
PotassiumSodiumSilicate
인화 인듐
실리콘 카바이드
Magnesium fluorosilicate
플루오로규산
attapulgite clay
실리콘 분
Praseodymium-Zircon Yellow
Premixing agent
Silica gel,microporous roundness
polyimide adhesive YJ-5
포스포러스옥시클로라이드
플루오르화칼슘
C.I. 색소 청색 29
헥사불화규산 마그네슘
울라스토니트
Porcelain glaze,industrial
molecular sieve type Ca—Y
규산소다
멜라민
황산 스트론튬
삼염화 인
실리카, 무정형 수화
규화불화 나트륨
저마늄(게르마늄)
인화갈륨
Chrom-tin-Pink Stannite Pigment
molecular sieve.Rare-earths
메타규산 나트륨, 펜타히드레이트
Phosphate rock and Phosphorite, calcined
FNG silica gel
HEXAMETHYLBENZENE
규산칼륨
칼슘 인산염, 디베이직, 이염화물
실릭산, 납염
해사 공급 업체
글로벌( 577)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12453 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21695 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9352 | 55 |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 |
sales@tjzxchem.com | China | 559 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 |
hjt@hong-jin.com | China | 228 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |