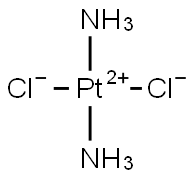시스백금
|
|
시스백금 속성
- 녹는점
- 270 °C (lit.)
- 밀도
- 3,7 g/cm3
- 저장 조건
- 2-8°C
- 용해도
- DMF에 용해됩니다. 가장 일반적인 용매에 불용성
- 물리적 상태
- 수정 같은
- 색상
- 노란색
- 수용성
- 19ºC에서 <0.1g/100mL
- Merck
- 14,2317
- 노출 한도
- ACGIH: TWA 0.002 mg/m3
NIOSH: IDLH 4 mg/m3; TWA 0.002 mg/m3
- 안정성
- 안정적인. 산화제, 알루미늄, 산화 방지제와 호환되지 않습니다.
- CAS 데이터베이스
- 15663-27-1(CAS DataBase Reference)
- IARC
- 2A (Vol. 26, Sup 7) 1987, 1 (Vol. 76, 100A) 2012
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 45-25-41-60-46-42/43-36/37/38 | ||
| 안전지침서 | 53-26-39-45-99-36/37/39-22 | ||
| 유엔번호(UN No.) | UN 3288 6.1/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TP2455000 | ||
| F 고인화성물질 | 10-21 | ||
| TSCA | Yes | ||
| HS 번호 | 2843 90 90 | ||
| 위험 등급 | 6.1(a) | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 15663-27-1(Hazardous Substances Data) | ||
| 독성 | LD50 in guinea pigs: 9.7 mg/kg i.p. (Fleishman) | ||
| 기존화학 물질 | KE-05-0360 |
시스백금 C화학적 특성, 용도, 생산
화학적 성질
Cisplatin is a white powder or yellow crystalline solid with the melting point 268-272°C (decomposition). It is slightly soluble in water and easily soluble in dimethylformamide. In aqueous solution, it can be gradually transformed into trans-and hydrolysis.용도
Cisplatin is a cytostatic agent and it is used to treat various cancer types, including cancer of ovary, testis, lung, head, neck, bladder, neuroblastoma, and nephroblastoma, and Hodgkin’s disease and non-Hodgkin lymphoma.생산 방법
Cisplatin is obtained by the method described by Kauffman and Cowan, in which potassium(II) tetrachloroplatinate is treated with buffered aqueous ammonia solution. Pure cisplatin is obtained by recrystallization from dilute hydrochloric acid.Indications
Cisplatin (Platinol) is an inorganic coordination complex with a broad range of antitumor activity. It is especially useful in the treatment of testicular and ovarian cancer. It binds to DNA at nucleophilic sites, such as the N7 and O6 of guanine, producing alterations in DNA structure and inhibition of DNA synthesis. Adjacent guanine residues on the same DNA strand are preferentially cross-linked. This platinating activity is analogous to the mode of action of alkylating agents. Cisplatin also binds extensively to proteins. It does not appear to be phase specific in the cell cycle.정의
ChEBI: Cisplatin is a diamminedichloroplatinum compound in which the two ammine ligands and two chloro ligands are oriented in a cis planar configuration around the central platinum ion. An anticancer drug that interacts with, and forms cross-links between, D A and proteins, it is used as a neoplasm inhibitor to treat solid tumours, primarily of the testis and ovary.일반 설명
An anticancer drug. Orange-yellow to deep yellow solid or powder.공기와 물의 반응
Insoluble in water.반응 프로필
Cisplatin is incompatible with oxidizing agents. Cisplatin is also incompatible with aluminum. Cisplatin may react with sodium bisulfite and other antioxidants.화재위험
Flash point data for Cisplatin are not available; however, Cisplatin is probably combustible.Pharmaceutical Applications
CDDP, also referred to as cisplatinum or cisplatin, is a yellow powder and has found widespread use a chemotherapeutic agent.생물학적 활성
Cisplatin is a platinum-containing compound that acts as a DNA-crosslinking agent and interferes with replication and transcription, culminating in apoptosis. It forms intra- and interstrand crosslinks with DNA with intrastrand guanine-to-guanine or guanine-to-alanine links accounting for the majority of DNA binding. Cisplatin halts the cell cycle at the G2/M phase in vitro and is active against murine tumors transplanted into mice and in mouse xenograft models, including a reduction in tumor growth in a model of squamous cell carcinoma of the head and neck when administered at doses ranging from 7.5 to 12.5 mg/kg. Cisplatin also inhibits the RecA recombinase of M. tuberculosis (IC50 = 2 μM), blocking protein splicing and cell growth. Formulations containing cisplatin have been used, alone and in combination therapy, in the treatment of a variety of cancers.Mechanism of action
Cisplatin shows biphasic plasma decay with a distribution phase half-life of 25 to 49 minutes and an elimination half-life of 2 to 4 days. More than 90% of the drug is bound to plasma proteins, and binding may approach 100% during prolonged infusion. Cisplatin does not cross the blood-brain barrier. Excretion is predominantly renal and is incomplete.Clinical Use
Cisplatin, combined with bleomycin and vinblastine or etoposide, produces cures in most patients with metastatic testicular cancer or germ cell cancer of the ovary. Cisplatin also shows some activity against carcinomas of the head and neck, bladder, cervix, prostate, and lung.부작용
Renal toxicity is the major potential toxicity of cisplatin. Severe nausea and vomiting that often accompany cisplatin administration may necessitate hospitalization. Cisplatin has mild bone marrow toxicity, yielding both leukopenia and thrombocytopenia. Anemia is common and may require transfusions of red blood cells. Anaphylactic allergic reactions have been described. Hearing loss in the high frequencies (4000 Hz) may occur in 10 to 30% of patients. Other reported toxicities include peripheral neuropathies with paresthesias, leg weakness, and tremors. Excessive urinary excretion of magnesium also may occur.Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Poison by ingestion, intramuscular, submtaneous, intravenous, and intraperitoneal routes. Human systemic effects: change in audttory acuity, change in kidney tubules, changes in bone marrow, corrosive to skin, depressed renal function tests, hallucinations, nausea or vomiting. Experimental teratogenic and reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic fumes of Cland NOx. See also PLATINUM COMPOUNDS.잠재적 노출
A potential danger to those involved in the manufacture, formulation and administration of this anticancer chemotherapy agent. Contact with water causes decomposition.Carcinogenicity
Cisplatin is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.신진 대사
It is rapidly hydrated, resulting in a short plasma half-life of less than 30 minutes. It is eliminated predominantly via the kidney, but approximately 10% of a given dose undergoes biliary excretion. It is highly nephrotoxic and can cause significant damage to the renal tubules, especially in patients with preexisting kidney disease or one kidney or who are concurrently receiving other nephrotoxic drugs (e.g., cyclophosphamide or ifosfamide). Dosages should be reduced in any of the above situations. Clearance decreases with chronic therapy, and toxicities can manifest at a late date. To proactively protect patients against kidney damage, patients should be hydrated with chloride-containing solutions. Saline or mannitol diuretics can be administered to promote continuous excretion of the drug and its hydrated analogues. Sodium thiosulfate, which accumulates in the renal tubules, also has been used to neutralize active drug in the kidneys in an effort to avoid nephrotoxicity.운송 방법
UN2928 Toxic solids, corrosive, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material, Technical Name Required. UN3290 Toxic solid, corrosive, inorganic, n.o.s., Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materialsPurification Methods
Recrystallise it from dimethylformamide and check the purity by IR and UV-VIS spectroscopy. [Raudaschl et al. Inorg Chim Acta 78 143 1983.] HIGHLY TOXIC, SUSPECTED CARCINOGEN.비 호환성
Aluminum reacts with cisplatin and decreases the drug’s effectiveness. Do not use any aluminum equipment to prepare or administer cisplatin.폐기물 처리
Disposal of unused product must be undertaken by qualified personnel who are knowledgeable in all applicable regulations and follow all pertinent safety precautions including the use of appropriate protective equipment. For proper handling and disposal, always comply with federal, state, and local regulations참고 문헌
1) Van Waardenburg et al. (2004), Platinated DNA adducts enhance poisoning of DNA topoisomerase I by camptothecin; J. Biol. Chem,, 279 54502 DOI:10.1074/JBC.M4101032002) Siddik et al. (2003), Cisplatin: mode of cytotoxic action and molecular basis of resistance; Oncogene, 22 7265 DOI:10.1038/sj.onc.1206933
3) Seki et al. (2000), Cisplatin (CDDP) specifically induces apoptosis via sequential activation of caspase-8, -3 and -6 in osteosarcoma; Cancer Chemother. Pharmacol., 45 199 DOI:10.1007/s002800050030
4) Nomura et al. (2004), Cisplatin inhibits the expression of X-linked inhibitor of apoptosis protein in human LNCaP cells; Urol. Oncol., 22 453 DOI:10.1016/J.UROLONC.2004.04.035
5) Raghavan et al. (2015), Dimethylsulfoxide inactivates the anticancer effect of cisplatin against myelogenous leukemia cell lines in in vitro assays.; Indian J. Phamracol., 47 322 DOI:10.4103/0253-7613.157132
6) Synthesis of Essential Drugs (2006, Elsevier) - libgen.lc
7) Sittig's Pharmaceutical Manufacturing Encyclopedia
8) Patty's Toxicology 6-Volume Set-Wiley (2012)
9) Modern pharmacology with clinical applications (2004, LWW)
시스백금 준비 용품 및 원자재
원자재
준비 용품
시스백금 공급 업체
글로벌( 741)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| SHANDONG BOYUAN PHARMACEUTICAL CO., LTD. | +86-0531-69954981 +8615666777973 |
dwyane.wang@boyuanpharm.com | China | 211 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 893 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 |
product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| AB PharmaTech,LLC | 323-480-4688 |
United States | 989 | 55 | |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| TianYuan Pharmaceutical CO.,LTD | +86-755-23284190 13684996853 |
sales@tianpharm.com | CHINA | 304 | 58 |
시스백금 관련 검색:
바나듐(III) 아세틸아세토네이트 백금분 팔라듐 시스백금
Oxaliplatin
Carboplatin
Tetrakis(triphenylphosphine)palladium
Bis(diphenylphosphinophenyl)ether palladium (II) dichloride
Cisplatin Injection
carboquone
CIS-DIAMMINEDICHLOROPLATINUM(II),TRANS-DIAMMINEDICHLOROPLATINUM(II),trans-diamminedichloroplatinu
DIAMMINE PLATINUM(Ⅱ) CHLORIDE
cisplatin-deoxy(adenosine monophosphate guanosine) adduct
CIS-DIAMMINEDICHLOROPLATINUM, (CISPLATIN),CIS-DIAMMINEDICHLOROPLATINUM, (CISPLATIN)
deoxyadenylyl-thymidyl-deoxyguanylyl-deoxyguanosine-cisplatin complex
cisplatin-deoxy(guanosine monophosphate guanosine) adduct
DVP (cisplatin)
cisplatin-guanosine adduct