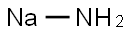나트륨아미드 C화학적 특성, 용도, 생산
화학적 성질
grey powder
물리적 성질
White crystalline powder with odor of ammonia; orthogonal crystals; density 1.39 g/cm
3; melts at 210°C; begins to volatilize at 400°C; decomposes at 500°C; decomposed by water and hot alcohol; in fused state it dissolves zinc, magnesium and other metals, as well as, quartz, glass, and silicates.
용도
Sodium Amide is widely used in organic chemical synthesis as a strong base in reactions such as in the preparation of phenylacetylene from 1,2-dibromophenylethane. Also used in the synthesis of 2,6-diamination of substituted pyridines as initiator of heterogeneous chichibabin reaction.
제조 방법
Sodium amide is prepared by passing dry ammonia gas over sodium metal at 350°C: 2Na + 2NH
3 → 2NaNH
2 + H
2Also, it may be prepared by reacting sodium metal with liquid ammonia in the presence of a catalyst such as iron(III) nitrate. The compound must be stored in well-sealed containers free from air or moisture.
정의
A
white ionic solid formed by passing dry
ammonia over sodium at 300–400°C. The
compound reacts with water to give
sodium hydroxide and ammonia. It reacts
with red-hot carbon to form sodium
cyanide and with nitrogen(I) oxide to form
sodium azide. Sodamide is used in the
Castner process and in the explosives industry.
화학 반응
Sodamide, sodamine, NaNH2, white solid, formed by reaction of sodium metal and dry NH3 gas at 350 °C (662 °F), or by solution of the metal in liquid ammonia. Reacts with carbon upon heating, to form sodium cyanide, and with nitrous oxide to form sodium azide, NaN3.
일반 설명
Odorless colorless solid. Denser than water.
공기와 물의 반응
Highly flammable. Reacts violently with water and bursts into flame.
반응 프로필
Sodium amide is a powerful reducing agent. Reacts violently with oxidizing agents. Reacts violently with steam and water to form caustic NaOH and NH3 vapors [Bergstrom et al., Chem. Reviews, 12:6 1932]. May form explosive compounds in the presence of water and carbon dioxide [Handling Chemicals Safely 1980 p 826]. Liable to deflagration upon heating and friction. Forms an explosive peroxide on storage. When Sodium amide and chromic anhydride are mixed together a vigorous reaction results; the same occurs with other oxidizing agents including dinitrogen tetraoxide, potassium chlorate, sodium nitrite. [Mellor 11:234 1946-47]. Reaction with 1,1-diethoxy-2- chloroethane produces sodium ethoxyacetylide, which is extremely pyrophoric [Rutledge 1968 p. 35]. Reactions with halogenated hydrocarbons may be violently explosive. Sodium amide forms toxic and flammable H2S gas with CS2. (714).
위험도
Flammable, dangerous fire risk.
건강위험
Ammonia gas formed by reaction of solid with moisture irritates eyes and skin. Solid causes caustic burns of eyes and skin. Ingestion burns mouth and stomach in same way as caustic soda and may cause perforation of tissue.
화재위험
Special Hazards of Combustion Products: Toxic and irritating ammonia gas may be formed.
Safety Profile
An intense irritant to tissue, skin, and eyes. Flammable by chemical reaction. Ignites or explodes with heat or grinding. Explosive reaction with moisture, chromium trioxide, potassium chlorate, halocarbons (e.g., 1,l -diethoxy-2chloroethane), oxidants, sodium nitrite, air. Can become explosive in storage. Violent reaction with dinitrogen tetraoxide. Will react with water or steam to produce heat and toxic and corrosive fumes of sodium hydroxide and ammonia. When heated to decomposition it emits highly toxic fumes of NH3 and Na2O. See also AMIDES.
Purification Methods
It reacts violently with H2O and is soluble in liquid NH3 (1% at 20o). It should be stored in wax-sealed containers in small batches. It is very hygroscopic and absorbs CO2 and H2O. If the solid is discoloured by being yellow or brown in colour, then it should be destroyed as it can be highly EXPLOSIVE. It should be replaced if discoloured. It is best destroyed by covering it with much toluene and slowly adding dilute EtOH with stirring until all the ammonia is liberated (FUME CUPBOARD). [Dennis & Bourne Inorg Synth I 74 1939, Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 465 1963, Bergstrom Org Synth Coll Vol III 778 1955.]
나트륨아미드 준비 용품 및 원자재
원자재
준비 용품
노넨(시스-6-)-1-올
1-ethynyl-2-methyl penten-2-ol
diethyl [[(6-methyl-2-pyridyl)amino]methylene]malonate
2-Amino-4-ethylpyridine
ETHYL 4-PYRIDYLACETATE
2-메틸인돌린
3-(o-chlorophenyl)-3-hydroxy-3-phenylpropiononitrile
ethyl 4-hydroxy-7-methyl-1,8-naphthyridine-3-carboxylate
4-PHENYL-PYRIDIN-2-YLAMINE
ACETOPHENAZINE MALEATE (200 MG)
Meclozine
Aprindine
2-Amino-4,6-dimethylpyridine
NORMEPERIDINE
FMOC-2-AMINONICOTINIC ACID
3-(P-CHLOROPHENYL)-3-(2-PYRIDYL)PROPYLALDEHYDE DIETHYL ACETAL
BEPRIDIL
4-Bromo-2,2-diphenylbutyric acid
4-CYANO-4-PHENYLPIPERIDINE HYDROCHLORIDE
(1-phenylcyclopropyl)methanamine
Setastine
2-Pyridinepropanol
FEMA 3082
CYCLOHEXYLPHENYLACETONITRILE
4-phenyl-1-(p-tolylsulphonyl)piperidine-4-carboxylic acid
ALPHA,ALPHA-DIPHENYL-GAMMA-BUTYROLACTONE
Loperamide
2-AMINO-3,5-DICHLORO-4,6-DIMETHYL PYRIDINE
(1-HYDROXY-CYCLOHEXYL)-ACETONITRILE
1,3-디(2,4,6-트리옥소헥사히드로-5-피리미딘일리덴)아이소인돌
3-CYCLOHEXYL-1-PROPYNE
PYRIDINE-4-ACETIC ACID
2,6-DIAMINO-4-METHYL PYRIDINE
2,6-DIMETHYL-3,5-HEPTANEDIONE
3-PROPYLISOXAZOL-5-AMINE
메틸페닐글리시드산에틸
4-PROPYL-PYRIDIN-2-YLAMINE
4-Phenylpiperidine
6-CHLORO-4-METHOXY-PYRIDINE-2-CARBOXYLIC ACID
1-methyl-4-phenylpiperidine-4-carbonitrile