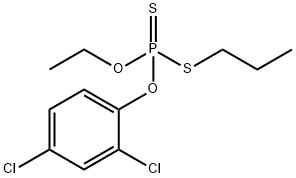
프로티오포스
|
|
프로티오포스 속성
- 녹는점
- 25°C
- 끓는 점
- 126.5 °C
- 밀도
- 1.3000
- 증기압
- 3.0×10-4 Pa (20 °C)
- 저장 조건
- APPROX 4°C
- 수용성
- 0.07mg l-1(20°C)
- 물리적 상태
- 액체
- BRN
- 1998314
- CAS 데이터베이스
- 34643-46-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-50/53 | ||
| 안전지침서 | 60-61 | ||
| 유엔번호(UN No.) | UN 3082 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TD5680000 | ||
| 기존화학 물질 | KE-10189 |
| 그림문자(GHS): |
 
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | ||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||||||||||||||||
| 예방조치문구: |
|
프로티오포스 C화학적 특성, 용도, 생산
개요
Prothiofos is a colorless liquid. It is nearly insoluble in water (1.7 mg/L at 20 ?C) but readily soluble in most organic solvents. Log Kow = 5.67. It is relatively stable in aqueous media; DT50 values (22 ?C) at pH 4, 7, and 9 are 120, 280, and 12 d, respectively.용도
Prothiofos is used to control chewing insects in a range of crops including vegetables, fruit, maize, sugar cane and ornamentals.정의
ChEBI: An organic thiophosphate that is the 2,4-dichlorophenyl ester of O-ethyl S-propyl dithiophosphoric acid.신진 대사 경로
A report by Nihon Tokushu Noyaku Seizo K.K. (1979) (now Nhon Bayer Agrochem K.K.) has summarised the nature of the photolysis products and metabolites formed by prothiofos in plants, insects, chicken, mice, rats, guinea-pigs and rabbits. Major routes for the metabolism of prothiofos include activation via oxidative desulfuration to the oxon and detoxification by dearylation to give 2,4-dichlorophenol which occur in all media. In addition, cleavage of the P-S bond and loss of the propanethiol moiety is an important detoxification mechanism in mammals but not insects and dechlorination by reductive loss of the 2-chlorine substituent in the phenyl ring occurs in soil, plants and photochemically. Stage II metabolism results in the formation of the glucoside of 2,4-dichlorophenol in plants and insects and the glucuronide and sulfate ester in mammals.신진 대사
The principal metabolic routes are activation by oxidative desulfuraton and detoxification by dearylation and cleavage of the P?S bond in both animals and plants. Prothiofos is strongly adsorbed in soil; the half-life under field conditions is 1–2 months.프로티오포스 준비 용품 및 원자재
원자재
준비 용품
프로티오포스 공급 업체
글로벌( 70)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 47465 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 |
sales@molcore.com | China | 49739 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 |
info@gihichemicals.com | China | 49999 | 58 |
| ShenZhen H&D Pharmaceutical Technology Co., LTD | +8613627253706 |
sale@hdimpurity.com | China | 1490 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 9309 | 58 |