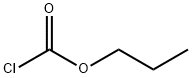
클로로탄산 n-프로필에스테르 C화학적 특성, 용도, 생산
화학적 성질
Propyl Chloroformate (PCF) is a colorless to yellow liquid, with a pungent odor. It reacts with water and spontaneous decomposes. It is miscible with benzene and ether.
용도
Propyl Chloroformate is used as a dramatizing agent for the determination of morphine, codeine and 6-acetyl morphine in urine and blood using direct aqueous derivatization.
생산 방법
Prepared by the reaction of liquid anhydrous n-propyl alcohol
with molar excess of dry, chlorine-free phosgene at low
temperature.
주요 응용
Propyl chloroformate has been used:
as derivatization reagent in quantification of dopamine, serotonin and norepinephrine in human urine by solid phase microextraction-gas chromatography-triple quadrupole mass spectrometry
in the preparation of dipropyl 3,6-diphenyl-1,2-dihydro-1,2,4,5-tetrazine-1,2-dicarboxylate
N-propyl chloroformate is an intermediate of the fungicide propamocarb.
제조 방법
Synthesis of Propyl chloroformate: Add n-propanol to the phosgene-passing kettle, start stirring, and then pass phosgene through the phosgene flowmeter. The phosgene-passing temperature is about 30 °C, and continue to pass the phosgene for several hours. When the end point is approached, sample and analyze, and stop when the standard is reached. Pass phosgene, cool, and then pass nitrogen to drive away phosgene and hydrogen chloride, destroy phosgene, and sample and analyze it as a product.
Reaction equation: CH3CH2CH2OH+COCl2→ClCOOCH2CH2CH3
일반 설명
N-propyl chloroformate appears as a colorless liquid. May be decomposed by water. Severely irritates skin and eyes. Very toxic by ingestion, inhalation or skin absorption. Denser than water and vapors heavier than air. Flash point 50°F. Used to make other chemicals.
공기와 물의 반응
Highly flammable. Water (moisture in air or soil) reacts with generation of heat and hydrochloric acid.
반응 프로필
Unstable, decomposes spontaneously to form hydrochloric acid and other products. Avoid moist air. [EPA, 1998]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
건강위험
Strongly irritating to eyes and mucous membranes. Poisonous; may be fatal if inhaled, swallowed or absorbed through skin.
화재위험
When heated to decomposition, Propyl chloroformate emits toxic fumes of chlorine containing compounds. Propyl chloroformate is a flammable/combustible material; Propyl chloroformate may be ignited by heat, sparks or flames. Vapors may travel to a source of ignition and flash back. Container may explode in heat or fire. Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Runoff from fire control or dilution water may cause pollution. Gradually decomposed by water and alcohol. Unstable, decomposes spontaneously to form hydrochloric acid and other products. Avoid moist air.
Safety Profile
Poison by skin contact.
Moderately toxic by ingestion and
inhalation. A corrosive irritant to skin, eyes,
and mucous membranes. Flammable when
exposed to heat or flame, can react
vigorously with oxidizing materials. When
heated to decomposition it emits toxic
fumes of Cl-. Used as a reactive intermediate
to polymerization initiators.
잠재적 노출
Propyl chloroformate is used in
organic synthesis; as an intermediate for polymerization
initiators; may have been used as a military poison gas.
운송 방법
UN2740 n-Propyl chloroformate, Hazard class:
6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable
liquid, 8-Corrosive material. Inhalation Hazard Zone B
비 호환성
Propyl chloroformate forms explosive
mixture with air. Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explo-
sions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, water and alcohols.
Reaction with water forms heat and hydrochloric acid.
Attacks some metals and coating in the presence of
moisture.
클로로탄산 n-프로필에스테르 준비 용품 및 원자재
원자재
준비 용품