Efavirenz suppliers
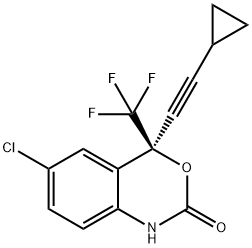
Efavirenz
- CAS:
- 154598-52-4
- MF:
- C14H9ClF3NO2
- MW:
- 315.67
Suppliers by country/region
Company Type
Properties
- Melting point:
- 139-141°C
- Boiling point:
- 340.6±42.0 °C(Predicted)
- alpha
- D20 -84.7° (c = 0.005 g/ml in CH3Cl); D25 -94.1° (c = 0.300 in methanol)
- Density
- 1.53±0.1 g/cm3(Predicted)
- Flash point:
- 2℃
- storage temp.
- -20°C
- solubility
- DMSO: soluble15mg/mL, clear
- pka
- 10.2(at 25℃)
- form
- powder or crystals
- color
- white to beige
- optical activity
- [α]/D -90 to -100°, c = 1 in methanol
- Water Solubility
- 8mg/L(temperature not stated)
- λmax
- 247nm(MeOH)(lit.)
- Merck
- 14,3521
- BCS Class
- 4
Safety Information
- Symbol(GHS)



GHS07,GHS08,GHS09
- Signal word
- Danger
- Hazard statements
- H302-H360-H410
- Precautionary statements
- P202-P264-P270-P273-P301+P312-P308+P313
- Hazard Codes
- N
- Risk Statements
- 50
- Safety Statements
- 61
- RIDADR
- UN3082 - class 9 - PG 3 - DOT NA1993 - Environmentally hazardous substances, liquid, n.o.s. HI: all (not BR)
- WGK Germany
- 3
- RTECS
- DM3440000
- HS Code
- 2934990002
Use
https://www.drugbank.ca/drugs/DB00625 Gallant JE, DeJesus E, Arribas JR, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354(3]:25160. Ena J, Pasquau F. Once-a-day highly active antiretroviral therapy: a systematic review. Clin Infect Dis. 2003;36(9]:118690. Vrouenraets SM, Wit FW, van Tongeren J, et al. Efavirenz: a review. Expert Opin Pharmacother. 2007;8(6]:85171. Efavirenz.[2009]. Sustiva. Rhodes, Greece: Cerner Multum. Date, H. L., Fisher, M.[2009]. HIV infection. In R. Walker and C. Whittlesea[eds.], Clinical Pharmacy and Therapeutics, 4th ed. London: Churchill Livingstone Elsevier, 568598 Wande, A. N.[1994]. Handbook of Pharmaceutical excipients, 2th ed. Washington, DC: American Pharmaceutical Association. Skidmore-Roth, L.[2009]. Mosbys Drug Guide of Nurses, 8th ed. St. Louis, MO: Mosby Elsevier, 346 pp. Almond, L. M., Hoggard, P. G., Edirisinghe, D. E., Khoo, S. H., Back, D. J.[2005]. Intracellular and plasma pharmacokinetics of efavirenz in HIV-infected individuals. Journal of Antimicrobial Chemotherapy, 56, 738744. Liu, P., Foster, G., LaBadie, R. R., Gutierrez, M. J., Sharma, A.[2005]. Pharmacokinetic interaction between voriconazole and efavirenz at steady state in healthy subjects. Clinical Pharmacology Therapeutics, 77, 40. https://www.rxlist.com/sustiva-drug.htm#indications Clercq, E.[2001]. Antiviral drugs: Current state of the art. Journal of Clinical Virology, 22, 7389. Langmann, P., Schirmer,D., Vath, T., Zilly,M., Klinker, H.[2001]. High-performance liquid chromatographic method for the determination of HIV-1 non-nucleoside reverse transcriptase inhibitor efavirenz in plasma of patients during highly active antiretroviral therapy. Journal of Chromatography. B, Biomedical Sciences and Applications, 755, 151156. YOUNG SD, BRITCHER SF, TRAN LO et al.: L-743, 726(DMP-266]: a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. (1995] 39(12]:2602-2605. DEEKS SG: International perspectives on antiretroviral resistance. Nonnucleoside reverse transcriptase inhibitor resistance. J. Acquir. Immune Defic. Syndr.[2001] 26[Suppl. 1]: S25-S33. BACHELER LT, ANTON ED, KUDISH P et al.: Human immunodeficiency virus type 1 mutations selected in patients failing efavirenz combination therapy. Antimicrob. Agents Chemother.[2000] 44(9]:2475-2484. JOLY V, DESCAMPS D, PEYTAVIN G et al.: Evolution of human immunodeficiency virus type 1(HIV-1] resistance mutations in nonnucleoside reverse transcriptase inhibitors[NNRTIs] in HIV-1-infected patients switched to antiretroviral therapy without NNRTIs. Antimicrob. Agents Chemother.[2004] 48(1]:172-175. Jena, A., Sachdeva, R. K., Sharma, A., Wanchu, A.[2009]. Adverse drug reactions to nonnucleoside reverse transcriptase inhibitorbased antiretroviral regimen: A 24-week prospective study. Journal of the International Association of Physicians in AIDS Care, 8, 318322. Paterson, D. L., Swindells, S., Brester, M., Vergis, E. N.[2000]. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine, 133, 2130. Arendt, G., Nocker,D., VonGiesen, H. J., Nolting, T.[2007].Neuropsychiatric side effects of efavirenz therapy. Expert Opinion on Drug Safety, 6, 147154. Lochet, P., Peyriere, H., Lotthe, A., Mauboussin, J. M., Delmas, B., Reynes, J.[2003]. Long-term assessment of neuropsychiatric adverse reactions associated with efavirenz. HIV Medicine, 4, 6266. OMahony, S. M., Myint, A., Steinbusch, H., Leonard, B. E.[2005]. Efavirenz induces depressive like behaviour, increased stress response and changes in the immune response in rats. Neuroimmunomodulation, 12, 293298. Romao, P. R. T., Lemos, J. C., Moreira, J., de Chaves, G., Moretti, M., Castro, A. A., et al.[2009]. Anti-HIV drugs nevirapine and efavirenz affect anxiety-related behavior and cognitive performance in mice. Neurotoxicity Research. Bickel, M., Stephan, C., Rottmann, C., Carlebach, A., Haberl, A., Kurowski, M., et al.[2005]. Severe CNS side effect and persistent high efavirenz plasma levels in a patient with HIV/HCV coinfection and liver cirrhosis. Scandinavian Journal of Infectious Diseases, 37, 520252. Gutierrez, F., Navarro, A., Padilla, S., Anton, R., Masia, M., Borras, J., et al.[2005]. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clinical Infectious Diseases, 41, 16481653. Treisman, G. J., Kaplan, A. I.[2002]. Neurologic and psychiatric complications of antiretroviral agents. AIDS, 16, 12011215. Hawkins, T., Geist, C., Young, B., Giblin, A., Mercier, R. C., Thornton,K., et al.[2005]. Comparison of neuropsychiatric side effects in an observational cohort of efavirenzand protease inhibitor-treated patients. HIV Clinical Trials, 6, 187196. 28. Blanch, J., Corbella, B., Garcia, F., Parellada, E., Gatell, J. M.[2001]. Manic syndrome associated with efavirenz overdose. Clinical Infectious Diseases, 15, 270271. Fumaz, C. R., Munoz-Moreno, J. A., Molt o, J., Negredo, E., Ferrer, M. J. M. A., Sirera, J., et al.[2005]. Long-term neuropsychiatric disorders on efavirenz-based approaches: Quality of life, psychologic issues, and adherence. Journal of Acquired Immune Deficiency Syndromes, 38, 560565. https://www.drugs.com/mtm/efavirenz.html Efavirenz D5 was launched as Sustiva in the US for the treatment of infection by HIV, the virus causing AIDS, in combination with other anti-retroviral agents. Efavirenz D5 is a non-nucleoside reverse transcriptase inhibitor (NNRTI) belonging to the 3,1-benzoxazin-2-one chemical class. It is the third non-nucleoside reverse transcriptase inhibitor to have been launched to date, after Nevirapine (1996) and Delavirdine (1997), increasing the arsenal of anti-HIV drugs for treating infected patients in dual or triple combination with nucleoside or other non-nucleoside RTIs, or protease inhibitors. Efavirenz D5 can be obtained by two related ways of six steps from 4-chloroaniline ; one of them is based on asymmetric synthesis by enantioselective addition of an acetylide to a trifluoroacetophenone. The anti-HIV activity of Efavirenz D5 was demonstrated against most wild-type and clinical strains of HIV-1, including those with the most frequently observed mutations. Efavirenz D5 has a better pharmacokinetic profile when compared with the preceding drugs of this class ; in particular, in a long-term experiment conducted in cynomolgus monkeys, Efavirenz D5 was shown to easily cross the blood brain barrier leading to an increase of the antiviral concentration in cerebrospinal fluid. Efavirenz D5 is a nonnucleoside HIV-1 reverse transcriptase inhibitor. Antiviral Efavirenz D5 was launched as Sustiva in the US for the treatment of infection by HIV, the virus causing AIDS, in combination with other anti-retroviral agents. Efavirenz D5 is a non-nucleoside reverse transcriptase inhibitor (NNRTI) belonging to the 3,1-benzoxazin-2-one chemical class. It is the third non-nucleoside reverse transcriptase inhibitor to have been launched to date, after Nevirapine (1996) and Delavirdine (1997), increasing the arsenal of anti-HIV drugs for treating infected patients in dual or triple combination with nucleoside or other non-nucleoside RTIs, or protease inhibitors. Efavirenz D5 can be obtained by two related ways of six steps from 4-chloroaniline ; one of them is based on asymmetric synthesis by enantioselective addition of an acetylide to a trifluoroacetophenone. The anti-HIV activity of Efavirenz D5 was demonstrated against most wild-type and clinical strains of HIV-1, including those with the most frequently observed mutations. Efavirenz D5 has a better pharmacokinetic profile when compared with the preceding drugs of this class ; in particular, in a long-term experiment conducted in cynomolgus monkeys, Efavirenz D5 was shown to easily cross the blood brain barrier leading to an increase of the antiviral concentration in cerebrospinal fluid. Efavirenz D5 (Sustiva)84 is also mandated for use with at leasttwo other antiretroviral agents. The compound is morethan 99% protein bound, and CSF concentrations exceedthe free fraction in the serum. Metabolism occurs in theliver. The half-life of a single dose of Efavirenz D5 is 52 to 76hours, and 40 to 55 after multiple doses (the drug inducesits own metabolism). Peak concentration is achieved in 3to 8 hours. Elimination is 14% to 34% in urine (as metabolites)and 16% to 41% in feces (primarily as Efavirenz D5).The oral dosage form is supplied as a capsule. Efavirenz D5 is a nonnucleoside HIV-1 reverse transcriptase inhibitor. Antiviral Efavirenz D5 is asynthetic heterocyclic compound formulated for oral administration. The most common (5%, moderate–severe) adverse effects associated with Efavirenz D5 therapy are rash, dizziness, nausea, headache, fatigue, insomnia and vomiting. Rash occurs in up to 26% of patients, mostly in the first 2 weeks of therapy. It usually resolves within 1 month, but is sufficiently severe to limit treatment in a few cases. Dizziness, insomnia, somnolence, impaired concentration, abnormal dreaming and other CNS disturbances have been reported in around 52% of clinical trial participants, with events of moderate to severe intensity occurring in about 3% of patients. Rare (0.2% of patients) episodes of severe delusional or inappropriate behavior and severe acute depression have also been reported. The symptoms commonly begin in the first 2 weeks of treatment but often resolve or substantially improve within a month. Elevations in serum hepatic transaminase to levels more than five times the upper limit of normal are observed in about 3% of patients and 8% of those co-infected with viral hepatitis B or C. Efavirenz D5 can be taken with or without food, but if taken with fatty food, its bioavailability and toxicity may increase. The oral solution has lower bioavailability than tablets or capsules. Efavirenz D5 is highly bound to human plasma proteins(approximately 99.7%), predominantly albumin[7]. It is principally metabolized by the cytochrome P450(CYP)system to hydroxylated metabolites with subsequent glucuronidation. These metabolites are essentially inactive against HIV-1. Efavirenz D5 drug has been shown to induce CYP enzymes, resulting in the induction of its own metabolism. Multiple doses of 200–400 mg per day for 10 days resulted in a lower-than predicted extent of accumulation(22%–42% lower)and a shorter terminal half-life of 40–55 hr(single-dose half life of 52–76 hr)6,7. Drug interaction studies, through in vivo and in vitro tests, demonstrated that EFV is able to induce or inhibit the CYP isoenzymes. Efavirenz D5 has been shown in vivo to cause hepatic enzyme induction, thus increasing the biotransformation of some drugs metabolized by CYP3A(human gene of CYP, family 3, subfamily A), including auto-induction of its own metabolism. The inducing effect on CYP3A is expected to be similar for Efavirenz D5 doses of 200–600 mg. In vitro, the 2C9, 2C19, and 3A4 isoenzymes were inhibited by Efavirenz D5. Co-administration of efavirenz with drugs primarily metabolized by 2C9, 2C19, and 3A4 iso-zymes may result in altered plasma concentrations of the co-administered drug. Drugs that induce CYP3A activity would be expected to increase the clearance of Efavirenz D5, resulting in lowered plasma concentrations[5, 7,9,10].
412 supplier list of "Efavirenz"
- Product Name:Efavirenz
- Products Intro:Purity: 0.99 | Package: 5KG;1KG/aluminum foil package; 25kg/drum | CustNote: Paypal,credit card,western union,bank transfer
- Company Type: Trader
- Country/Region: CHINA
- Main Products: hgh191aa,peptides,sarms
-
1mL*10mM(inDMSO)$50.0010mg$52.0025mg$70.0050mg$88.00
- Product Name:Efavirenz
- Products Intro:Brand:TargetMol | Product Number:T2393 | Purity:99.83%|99.58%|99.2%
- Company Type: Trader
- Country/Region: UNITED STATES
- Main Products: Compound Libraries,Noval Small Molecucles,Nature Compounds,Inhibitory Antibodies,Life science Kits