| | MERCURIC THIOCYANATE Basic information |
| Product Name: | MERCURIC THIOCYANATE | | Synonyms: | Quecksilber(II)-thiocyanat;Thiocyanic acid, mercury salt;Mercuric thiocyanate: (Mercuric sulfocyanate);MERCURY THIOCYNATE pure;Bisthiocyanatomercury(II);Bisthiocyanic acid mercury(II) salt;Mercury(II)bisthiocyanate;Mercury(II) thiocyan | | CAS: | 592-85-8 | | MF: | C2HgN2S2 | | MW: | 316.75 | | EINECS: | 209-773-0 | | Product Categories: | Chemistry;Inorganics | | Mol File: | 592-85-8.mol | 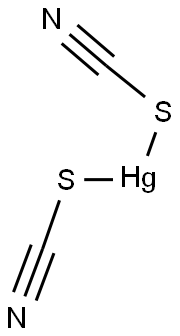 |
| | MERCURIC THIOCYANATE Chemical Properties |
| Melting point | 165 °C (dec.) (lit.) | | density | 3.71 g/mL at 25 °C (lit.) | | Fp | 120 °C | | storage temp. | Store at RT. | | solubility | Methanol (Slightly) | | form | Powder | | color | White to pale yellow | | Water Solubility | Soluble in dilute hydrochloric acid, potassium cyanide and ammonia. Slightly soluble in alcohol and ether. Insoluble in water. | | Sensitive | Hygroscopic | | Merck | 14,5890 | | BRN | 3561344 | | Stability: | Stable. Incompatible with strong acids. May be light or moisture sensitive. | | CAS DataBase Reference | 592-85-8(CAS DataBase Reference) | | EPA Substance Registry System | Mercuric thiocyanate (592-85-8) |
| | MERCURIC THIOCYANATE Usage And Synthesis |
| Description | Mercury thiocyanate is a white, odorless powder. Molecular weight= 316.79; Freezing/Melting point=about 165℃ (decomposes). Slightly soluble in cold water. | | Chemical Properties | Mercuric thiocyanate is an inorganic chemical substance. It is a stable solid at room temperature, and depending upon the purity, it appears as odourless white crystalline powder or grey. It is insoluble in water and denser than water and sinks in water. On decomposition, mercuric thiocyanate releases hazardous substances such as cyanide vapours, vapours of mercury, oxides of nitrogen (NO, NO2), and oxides of sulphur (SO2, SO3). Mercury thiocyanate has limited uses in chemical synthesis. | | Chemical Properties | Mercury thiocyanate is a white, odorless
powder. | | Uses | Mercury(II) thiocyanate is used as a precursor to potassium tris(thiocyanato)mercurate(II) and cesium tris(thiocyanato)mercurate(II). It is also used in the determination of chloride ions in water by UV-visible spectroscopy. Further, it acts as a catalyst for the addition of thiocyanic acid to alkynes. | | Uses | For Pharaoh's serpents (fireworks); intensifier in photography. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | MERCURIC THIOCYANATE decomposes into its elements at about 165°C. Burns readily in air to generate a coil of cohesive ash resembling a serpent (hence used in a firework: Pharaoh's serpents). Swells up to many times its original volume if heated [USCG, 1999]. Soluble in dilute hydrochloric acid [Merck]. Incompatible with strong oxidizing agents. | | Hazard | Highly toxic by ingestion, inhalation, and
skin absorption. | | Health Hazard | Mercuric thiocyanate causes severe eye and skin irritation with possible burns and causes digestive and respiratory tract irritation with possible burns. It may impair fertility, may cause harm to the unborn child, is harmful if inhaled, may cause allergic skin reaction, may cause kidney damage, may cause CNS effects, is light sensitive, and is a severe marine pollutant. Contact with acids liberates very toxic gas. The target organs include kidneys, CNS, reproductive system, eyes, and skin. | | Safety Profile | A poison by ingestion
and intraperitoneal routes. Moderately toxic
by skin contact. Thermally unstable and
decomposition may be vigorous. When
heated to decomposition it emits very toxic
fumes of Hg, NOx, SOx, and CN-. See also
MERCURY COMPOUNDS and
CYANATES. | | Potential Exposure | Mercury thiocyanate is used in
photography and fireworks. | | First aid | If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Antidotes and special procedures for medical personnel: The drug NAP (n-acetyl penicillamine) has been used to treat mercury poisoning, with mixed success. Note to physician: For severe poisoning BAL [British AntiLewisite, dimercaprol, dithiopropanol (C3H8OS2)] has been used to treat toxic symptoms of certain heavy metals poisoning including mercury. Although BAL is reported to have a large margin of safety, caution must be exercised, because toxic effects may be caused by excessive dosage. Most can be prevented by premedication with 1-ephedrine sulfate (CAS: 134-72-5). | | storage | Color Code—Blue: Health Hazard/Poison: Store in a secure poison location. Prior to working with this chemical you should be trained on its proper handling and storage. Store in tightly closed containers in a cool, wellventilated area away from light, heat, and acids, including fumes. | | Shipping | UN1646 Mercury thiocyanate, Hazard Class:
6.1; Labels: 6.1-Poisonous materials. | | Purification Methods | Recrystallise it from H2O, and it can give various crystal forms depending on conditions. Its solubility in H2O is 0.069% at 25o, but is more soluble at higher temperatures. It decomposes to Hg above 165o. Poisonous. [Mason & Forgeng J Phys Chem 35 1121 1931, Birckenbach & Kolb Chem Ber 68 919 1935.] | | Incompatibilities | Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. Mercury thiocyanate is
sensitive to heat; expands to many times its original volume
and then decomposes at freezing/melting point forming
toxic fumes of sulfur oxides, mercury cyanide, and nitrogen
oxides. Contact with acid or acid fumes causes release
of toxic mercury and cyanide vapors. Incompatible with
chlorine, reducing agents such as hydrides, sulfides | | Waste Disposal | Small amounts may be
destroyed by alkaline hydrolysis. Admixture with alkali
can be followed by soil burial. Larger quantities can be
disposed of by incineration in admixture with acetone or
xylene and using effluent gas scrubbing. Do not reuse
empty container; proper disposal required. |
| | MERCURIC THIOCYANATE Preparation Products And Raw materials |
|