- Magnesium dioxide
-

- $15.00 / 1KG
-
2021-08-11
- CAS:14452-57-4
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Magnesium dioxide
-

- $1.00 / 1PCS
-
2021-07-06
- CAS:14452-57-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
|
| | Magnesium dioxide Basic information |
| Product Name: | Magnesium dioxide | | Synonyms: | Magnesium dioxide;MAGNESIUM PEROXIDE LIGHT;MAGNESII PEROXIDUM;magnesiumperoxide(mg(o2));MAGNESIUM PEROXIDE LIGHT, PH HELV;Magnesium peroxide light, Ph Eur;MAGNESIUM PEROXIDE FOR ANALYTICAL PURPOSE 24-28 % AS MGO2;24-28%asMgO2 | | CAS: | 14452-57-4 | | MF: | MgO2 | | MW: | 56.3 | | EINECS: | 238-438-1 | | Product Categories: | Oxidation;Peroxides;Synthetic Reagents | | Mol File: | 14452-57-4.mol | 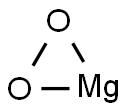 |
| | Magnesium dioxide Chemical Properties |
| | Magnesium dioxide Usage And Synthesis |
| Description | Magnesium peroxide is a white, odorless crystalline solid. Molecular weight= 56.31 (MgO2); 40.3(MgO); Decomposes above 100℃. Insoluble in water. | | Chemical Properties | Magnesium peroxide is a white, odorless
crystalline solid. | | Chemical Properties | White or slightly yellow, amorphous, light powder. | | Uses | Bleaching and oxidizing agent, medicine
(antacid). | | Uses | Magnesium peroxide is used mainly in medicine for treating hyperacidity in the gastric intestinal tract, and in the treatment of metabolic diseases such as diabetes and ketonuria. It is also used in the preparation of toothpaste and antiseptic ointments. All of these uses involve a mixture of magnesium peroxide, magnesium oxide, magnesium hydroxide, and an admixture of magnesium carbonate. Magnesium peroxide is also used in bleaching and agricultural applications. | | General Description | A white powder. Noncombustible but accelerates the burning of combustible material, if the combustible material is finely divided the mixture may be explosive. Mixtures of combustible material and the peroxide can be ignited by friction or contact with moisture. Used in medicine, and as a bleaching agent. | | Air & Water Reactions | Insoluble in water and slowly decomposed by water, liberating oxygen. | | Reactivity Profile | Mixtures of combustible material and the peroxide can be ignited with friction or moisture [AAR 1991]. Water gradually decomposes Magnesium dioxide liberating oxygen, with dilute acids Magnesium dioxide forms hydrogen peroxide, when strongly heated Magnesium dioxide losses all peroxide oxygen [Merck 11th ed. 1989]. | | Hazard | Powerful oxidizer and dangerous fire risk,
reacts with acidic materials and moisture. | | Health Hazard | Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard. | | Safety Profile | A powerful oxidizer.
Probably a severe irritant to the eyes, skin,
and mucous membranes. Flammable by
chemical reaction with acidic materials and
moisture; an oxidizing agent. Dangerous;
reacts vigorously with reducing agents; will
decompose violently in or near a fire. See
also MAGNESIUM COMPOUNDS and
PEROXIDES, INORGANIC. | | Potential Exposure | Magnesium peroxide is used as a
bleaching and oxidizing agent, and in the manufacture of
antacids and antiinfective drugs. | | First aid | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | | storage | Color Code—Yellow: Reactive Hazard; Store in alocation separate from other materials, especially flammables and combustibles. Prior to working with this chemical you should be trained on its proper handling andstorage. Magnesium peroxide must be stored to avoid contact with acids, since violent reactions occur. Store intightly closed containers in a cool, well-ventilated areaaway from flammable and combustible materials. Keepmagnesium peroxide dry. In contact with moisture, it is adangerous fire hazard because it releases oxygen and muchheat. See OSHA Standard 1910.104 and NFPA 43A Codefor the Storage of Liquid and Solid Oxidizers for detailedhandling and storage regulations. | | Shipping | UN1476 Magnesium peroxide, Hazard Class:
5.1; Labels: 5.1-Oxidizer. | | Incompatibilities | Powerful oxidizer. Dangerous fire risk
with flammable and combustible materials. Violent reaction
with acids. Keep away from moisture; causes the release of
oxygen and heat. |
| | Magnesium dioxide Preparation Products And Raw materials |
|