- Cefetamet hydrochloride
-

- $15.00 / 1KG
-
2021-08-12
- CAS:65052-63-3
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Cefetamet hydrochloride
-

- $0.00 / 1Kg
-
2020-05-25
- CAS:65052-63-3
- Min. Order: 1KG
- Purity: 99.0%+
- Supply Ability: 300 MT
|
| | Cefetamet hydrochloride Basic information | | Synthesis |
| Product Name: | Cefetamet hydrochloride | | Synonyms: | DEACETOXYCEFOTAXIME;(6R,7R)-7-[[(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid hydrochloride;Cefetamet hydrochloride;Ceftamet;[6R-[(6α,7β(Z)]]-7-[[(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;Ro-15-8074;(6R,7R)-7-[[(Z)-(2-Aminothiazole-4-yl)(methoxyimino)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]octa-2-ene-2-carboxylic acid;FR-13300 | | CAS: | 65052-63-3 | | MF: | C14H15N5O5S2 | | MW: | 397.43 | | EINECS: | 675-838-9 | | Product Categories: | | | Mol File: | 65052-63-3.mol | 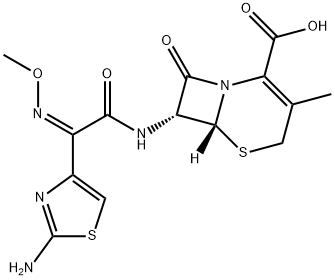 |
| | Cefetamet hydrochloride Chemical Properties |
| Melting point | >178°C (dec.) | | density | 1.83±0.1 g/cm3(Predicted) | | storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere | | solubility | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) | | pka | 3.11±0.50(Predicted) | | form | Solid | | color | White to Off-White | | Stability: | Hygroscopic | | CAS DataBase Reference | 65052-63-3(CAS DataBase Reference) |
| | Cefetamet hydrochloride Usage And Synthesis |
| Synthesis | Suspend 2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetic acid in tetrahydrofuran, add half amount of phosphorus oxychloride dropwise at 0 °C, keep the temperature not more than 5 Chemicalbook °C, dropwise The reaction was completed for 30 minutes. Dimethylformamide and the other half of phosphorus oxychloride were added, and the reaction was carried out at 0 °C for 1 h to obtain a solution of active ester (I) at -5 °C.
7-ADCA, bis-trimethylsilyl urea and ethyl acetate were refluxed at 75-77 °C for 2 h to obtain a solution of 7-ADCA silicon ester, which was cooled to -5 °C. It was combined with the solution of active ester and stirred at -5~0°C Chemicalbook for 2h. Be raised to 15 ℃, add water, stir for 30min. Separate the water layer, adjust to Ph=2.6~3.0 with sodium hydroxide solution, place to separate out crystallization. Filtration, acetone recrystallization to give Cefetamet, yield 82%. | | Description | Ceftazidime hydrochloride is an oral third-generation cephalosporin, which has the same antibacterial activity against gram-positive bacteria as cefixime, and is superior to cefaclor, especially against Streptococcus pneumoniae and Streptococcus pyogenes. It has obvious antibacterial activity against a variety of gram-negative bacteria such as Escherichia coli, Proteus, Klebsiella pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae, especially against Serratia, indole-positive Proteus, Enterobacter The antibacterial activity of Citrobacter and Citrobacter was significantly enhanced. | | Uses | Cefetamet is a third-generation cephalosporin antibiotic and the active agent of Cefeamet pivoxil (C242780) after hydrolysis. | | Definition | ChEBI: Cefetamet is a cephalosporin compound having methyl and [(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino side-groups; a cephalosporin antibiotic active against Neisseria gonorrhoeae. | | Antimicrobial activity | A semisynthetic cephalosporin formulated for oral use as the
prodrug ester, cefetamet pivoxyl. It is less active than cefaclor
and cefadroxil against Staph. aureus, but as active against
streptococci and more active against enterobacteria, H. influenzae
and N. gonorrhoeae, including β-lactamase-producing
strains. L. monocytogenes, C. difficile, Sten. maltophilia and Burk.
cepacia are all resistant. It is resistant to hydrolysis by common
plasmid-mediated enzymes.
The absolute bioavailability is about 50%. The plasma peak
is delayed by food. Binding to plasma protein is about 20%.
The volume of distribution approximates to the extracellular
water. It is excreted into urine with a half-life of 2–2.5 h,
principally in the glomerular filtrate. Elimination is linearly
related to creatinine clearance. Side effects and uses are similar
to those of other group 5 cephalosporins. |
| | Cefetamet hydrochloride Preparation Products And Raw materials |
|