Nysted Reagent manufacturers
- Geneticin
-

- $1.00 / 1KG
-
2019-07-06
- CAS:41114-59-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 25kg
|
| | Nysted Reagent Basic information |
| Product Name: | Nysted Reagent | | Synonyms: | Nysted Reagent,cyclo-Dibromodi-μ-methylene[μ-(tetrahydrofuran)]trizinc;Nysted Reagent, 20% w/w suspension in THF;Nysted reagent (20% wet suspension in THF);Nysted Reagent 20 wt. % suspension in THF;Nysted Reagent, 20% solution in THF, SpcSeal;Nystead reagent;bis(bromozinciomethyl)zinc (20% tetrahydrofuran solution);Nysted reagent 20% THF | | CAS: | 41114-59-4 | | MF: | C6H12Br2OZn3 | | MW: | 456.11 | | EINECS: | | | Product Categories: | API intermediates | | Mol File: | 41114-59-4.mol | 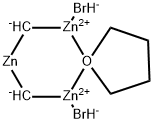 |
| | Nysted Reagent Chemical Properties |
| density | 1.186 g/mL at 25 °C(lit.) | | Fp | −15 °F | | InChIKey | CCTHTLJWXPUNGT-UHFFFAOYSA-L | | CAS DataBase Reference | 41114-59-4 |
| | Nysted Reagent Usage And Synthesis |
| Chemical Properties | The Nysted reagent is a reagent used in organic synthesis for the methenylation of a carbonyl group. It was discovered in 1975 by Leonard N. Nysted in Chicago, Illinois. It was originally prepared by reacting dibromomethane and activated zinc in THF.
A similar reagent is Tebbe's reagent.In the Nysted olefination, the Nysted reagent reacts with TiCl to methylenate a carbonyl group. The biggest problem with these reagents are that the reactivity has not been well documented. It is believed that the TiCl acts as a mediator in the reaction. Nysted reagent can methylenate different carbonyl groups in the presence of different mediators. For example, in the presence of BF•OEt, the reagent will methylenate aldehydes. On the other hand, in the presence of TiCl, TiCl or TiCl and BF•OEt, the reagent can methylenate ketones. Most commonly, it is used to methylenate ketones because of their general difficulty to methylenate due to crowding around the carbonyl group. The Nysted reagent is able to overcome the additional steric hinderance found in ketones, and more easily methylenate the carbonyl group.
There is little research on Nysted reagent because of the hazards and high reactivity and the difficulty of keeping the reagent stable while it is in use. More specifically, it can form explosive peroxides when exposed to air and is extremely flammable. Also, it reacts violently with water. These make this reagent very dangerous to work with. | | Uses | Nysted Reagent is a reagent used in synthetic reactions such as the synthesis of Entcavir. | | Uses | The cyclo-dibromo-di-m-methylene-(m-tetrahydrofuran)-trizinc is known as the Nysted reagent. This reagent is prepared by treatment of dibromomethane with activated zinc-lead couple or hydrogen chloride-activated zinc dust in THF. This reagent is useful for converting a carbonyl group into a methylene group, and such transformation is known as the Nysted methylenation or Nysted olefination. The study finds that this reagent is superior to the Tebbe reagent. | | Uses | Reagent for the methylenation of ketones and aldehydes. | | Application | Useful reagent for the methylenation of ketones and α-ketols.
Reactant for:
Synthesis of amphidinolide T3 using a ring-closing metathesis and asymmetric dihydroxylation strategy
Methylenation of carbonyl compounds
Olefination reactions | | Reactions | 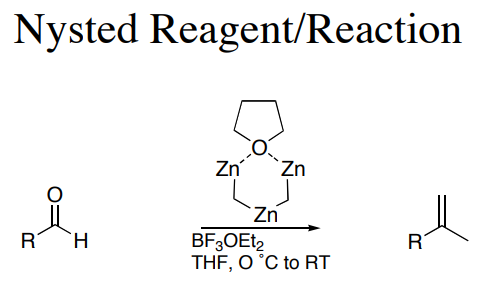
This commerically available reagent is capable of methyenylation alone or in concert with TiCl4. While information on the reactivity of the reagent exists, the mechanistic basis of its function has yet to be elucidated. |
| | Nysted Reagent Preparation Products And Raw materials |
|