Barium iodide manufacturers
- Barium iodide
-

- $40.00 / 1kg
-
2023-09-26
- CAS:13718-00-8
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10 tons
- Barium iodide
-

- $0.00 / 1KG
-
2023-09-06
- CAS:13718-00-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- Barium iodide
-

- $0.01 / 1KG
-
2020-08-12
- CAS:13718-00-8
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 50 tons
|
| | Barium iodide Basic information |
| Product Name: | Barium iodide | | Synonyms: | BARIUM IODIDE;4,4'-Bi-3H-dinaphtho[2,1-d:1',2'-f][1,3]diazepine;3H,3'H-[4,4']bi[dinaphtho[2,1-d,1',2'-f][1,3]diazepinyl | | CAS: | 13718-00-8 | | MF: | C42H26N4 | | MW: | 586.68 | | EINECS: | 237-276-9 | | Product Categories: | | | Mol File: | 13718-00-8.mol | 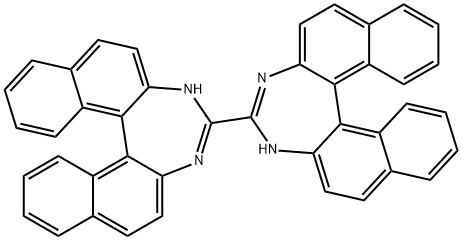 |
| | Barium iodide Chemical Properties |
| | Barium iodide Usage And Synthesis |
| Chemical Properties | Colorless crystals, decomposes and reddens on exposure to air.Soluble in water; slightly soluble in alcohol. | | Physical properties | Barium iodide occurs in two forms, one anhydrous
and the other as a dihydrate. Both are white solids. This salt is stable, but air and light sensitive. It is also
hygroscopic and moisture sensitive. Barium iodide is
incompatible with strong oxidizing agents. | | Uses | Preparation of other iodides. | | Preparation | Barium
iodide is made by the neutralization of barium
carbonate with HI in water:
BaCO3+2HI (aq)→BaI2 (aq)
It is also made by the reaction of BaH2 with ammonium
iodide in pyridine:
BaH2+2NH4I (aq)→BaI2 (aq)+ 2NH3 (gas)+H2 (gas)
The product crystallizes as the hydrate BaI2·2H2O. If
this heated, dehydration to anhydrous BaI2 occurs. The
dihydrate dehydrates at 539°C to form BaI2. |
| | Barium iodide Preparation Products And Raw materials |
|