|
|
| | ETHYL 4-CHLORO-2-QUINAZOLINECARBOXYLATE Basic information |
| Product Name: | ETHYL 4-CHLORO-2-QUINAZOLINECARBOXYLATE | | Synonyms: | 4-Chloro-2-(ethoxycarbonyl)quinazoline;ETHYL 4-CHLORO-2-QUINAZOLINECARBOXYLATE;Ethyl 4-chloroquinazoline-2-carboxylate;2-Quinazolinecarboxylic acid, 4-chloro-, ethyl ester;2-Quinazolinecarboxylic acid, 4-chloro-, ethyl ester (9CI, ACI);4-chloro-2-Quinazolinecarboxylic acid ethyl ester (9CI ACI) | | CAS: | 34632-69-4 | | MF: | C11H9ClN2O2 | | MW: | 236.65 | | EINECS: | | | Product Categories: | CHIRAL CHEMICALS | | Mol File: | 34632-69-4.mol | 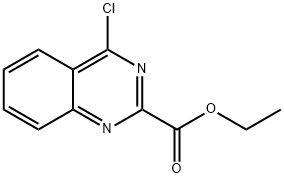 |
| | ETHYL 4-CHLORO-2-QUINAZOLINECARBOXYLATE Chemical Properties |
| Melting point | 100-101°C | | Boiling point | 391.3±34.0 °C(Predicted) | | density | 1.341±0.06 g/cm3(Predicted) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | form | solid | | pka | -0.74±0.30(Predicted) | | Appearance | White to yellow Solid | | InChI | InChI=1S/C11H9ClN2O2/c1-2-16-11(15)10-13-8-6-4-3-5-7(8)9(12)14-10/h3-6H,2H2,1H3 | | InChIKey | ZMAJSODACDWUAS-UHFFFAOYSA-N | | SMILES | N1=C2C(C=CC=C2)=C(Cl)N=C1C(OCC)=O |
| Hazard Codes | Xi,Xn | | Risk Statements | 22 | | HazardClass | IRRITANT | | HS Code | 2933599590 |
| | ETHYL 4-CHLORO-2-QUINAZOLINECARBOXYLATE Usage And Synthesis |
| Uses | Ethyl 4-Chloroquinazoline-2-carboxylate is a reactant used in the synthesis and evaluation of novel quinazoline-based anti-inflammatory agents acting as PDE4B inhibitors. | | Synthesis | General procedure for the synthesis of ethyl 4-chloroquinazoline-2-carboxylate from ethyl 4-quinazolinone-2-carboxylate: To a suspension of ethyl 4-quinazolinone-2-carboxylate (1.2 g, 5.5 mmol) in chloroform (20 mL) was added sequentially N,N-dimethylformamide (6 drops) and thionyl chloride (4.8 mL, 66 mmol). The reaction mixture was heated to reflux for 4.5 hours. After completion of the reaction, it was cooled to room temperature and concentrated under reduced pressure to remove the solvent. Ice water was added to the residue and a white solid precipitated. The solid product was collected by filtration, washed with cold water and dried to afford ethyl 4-chloroquinazoline-2-carboxylate (1.26 g, 97% yield). The product was confirmed by 1H NMR (400 MHz, CDCl3): δ 8.38 (dd, J = 0.74 Hz, 8.34 Hz, 1H), 8.31 (d, J = 8.48 Hz, 1H), 8.07 (m, 1H), 7.87 (m, 1H), 4.61 (q, J = 7.12 Hz, 2H), 1.50 (t, J = 7.14 Hz, 3H).IR spectra (diamond cell, cm-1 ) showed characteristic absorption peaks: νmax 1731.3, 1549.8, 1479.8, 1338.4, 1179.4, 913.0, 764.3, 693.9.Melting point is 86-88 °C. | | References | [1] Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 18, p. 5045 - 5048
[2] Bioorganic and Medicinal Chemistry, 2015, vol. 23, # 8, p. 1701 - 1715
[3] Patent: WO2004/14844, 2004, A2. Location in patent: Page 187-188
[4] Chemical and Pharmaceutical Bulletin, 2015, vol. 63, # 2, p. 102 - 116 |
| | ETHYL 4-CHLORO-2-QUINAZOLINECARBOXYLATE Preparation Products And Raw materials |
|