| Company Name: |
Shanghai Rechem science Co., Ltd.
|
| Tel: |
21-31433387 15618786686 |
| Email: |
sales@rechemscience.com |
| Products Intro: |
CAS:34161-23-4
Purity:98% Package:10g;100g
|
|
| | FIPEXIDE HYDROCHLORIDE Basic information |
| Product Name: | FIPEXIDE HYDROCHLORIDE | | Synonyms: | 1-[2-(4-CHLOROPHENOXY)ACETYL]-4-[3,4-METHYLENEDIOXYBENZYL]PIPERAZINE HYDROCHLORIDE;FIPEXIDE HYDROCHLORIDE;1-(1,3-benzodioxol-5-ylmethyl)-4-[(4-chlorophenoxy)acetyl]piperazinium chloride;1-(1,3-Benzodioxol-5-yl-methyl)-4-[(4-chlorophenoxy)acetyl]piperazine hydrochloride;Attentil;BP-662;Vigilor;1-[4-(1,3-benzodioxol-5-ylmethyl)piperazin-1-yl]-2-(4-chlorophenoxy)ethanone hydrochloride | | CAS: | 34161-23-4 | | MF: | C20H22Cl2N2O4 | | MW: | 425.31 | | EINECS: | 2518569 | | Product Categories: | Miscellaneous Compounds | | Mol File: | 34161-23-4.mol | 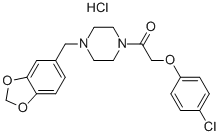 |
| | FIPEXIDE HYDROCHLORIDE Chemical Properties |
| Melting point | 230-232° | | storage temp. | -20°C Freezer, Under inert atmosphere | | solubility | DMSO (Slightly), Methanol (Slightly, Heated) | | form | Solid | | color | White to Off-White | | Water Solubility | Water: Insoluble |
| WGK Germany | 3 | | Toxicity | LD50 in Swiss mice, Sprague-Dawley rats, Wistar rats (mg/kg): 4150, 4482, 7000 orally; 499, 537, 450 i.p. (David) |
| | FIPEXIDE HYDROCHLORIDE Usage And Synthesis |
| Originator | Attentil,Ravizza | | Uses | Fipexide Hydrochloride is one of five potential drug candidates to overcome the melphalan-induced vascular toxicity. | | Manufacturing Process | 25.8 g (0.3 moles) of anhydrous piperazine and 32.5 ml (1.8 moles) of
distilled water (or simply 58.3 g (0.3 moles) of piperazine hexahydrate) are
loaded into a 250 ml flask provided with an agitator, a thermometer and a
reflux condenser, together with 51.2 g (0.3 moles) of piperonyl chloride,
whereupon 2 g of cetyltrimethylammonium bromide are added to the mixture
with vigorous agitation, and the flask is cooled with water so that the
temperature of the reaction mass under agitation does not rise above 110°C.
Once the exothermic stage is exhausted, the temperature is maintained at
130°C by an external oil bath, for 90 min under agitation.
After cooling, a solid mass is obtained which is taken up with 400 ml of an
aqueous solution containing 10% by weight of caustic soda to dissolve the
product from the mass. The alkaline solution thus obtained is extracted twice
with 500 ml of chloroform. The extract is washed with water and then
evaporated to dryness. The residue is crystallised from 96% ethanol.
50.5 g (theoretical value 53.18 g) of 1,4-bispiperonylpiperazine as a paleyellowish
white crystals are obtained with a melting point of 155-156°C; the
hydrochloride melts with decomposition above 260°C.
Preparation of fipexidum hydrochloride:
106.3 g (0.3 moles) of 1,4-bispiperonylpiperazine are dissolved in 750 ml of
hot benzene in a 2,000 ml flask provided with a stirrer and a reflux condenser
and 38 g (0.45 moles) of dry, powdered sodium bicarbonate are added. After
cooling to ambient temperature, 92.3 g (0.45 moles, corresponding to 63 ml)
of the chloride of p-chlorophenoxyacetic acid are added slowly, with agitation,
and the mixture is heated under reflux for 7 hours. The benzene is then
almost totally recovered by distillation at atmospheric pressure and the
residue is evaporated to dryness under vacuum. The solid residue thus
obtained is taken up with 400 ml of aqueous solution at 10% sodium
hydroxide (1 mole) and the alkaline liquid phase is extracted twice with 600
ml of chloroform. The chloroform extracts are joined together and washed
with a little water and then agitated vigorously with a solution of 200 ml of
concentrated hydrochloric acid in 300 ml of water. An abundant white
precipitate is obtained. After filtration under vacuum, the precipitate is treated
with boiling ethanol; the 1-((p-chlorophenoxy)acetyl)-4-piperonylpiperazine
hydrochloride (fipexide hydrochloride) passes into solution while the
dihydrochloride of hydrochloric acid remains undissolved and is separated by
filtration while hot. The alcoholic filtrate is cooled, with consequent slow
crystallization of the fipexide hydrochloride. 70.2 g of the product are obtainedwith a yield of 55%; melting point is (in Kofler) 228-230°C. | | Therapeutic Function | Antidepressant, Psychostimulant |
| | FIPEXIDE HYDROCHLORIDE Preparation Products And Raw materials |
|