| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
4008200310 |
| Email: |
marketing@tsbiochem.com |
| Products Intro: |
Product Name:Motretinide;Motretinide
CAS:56281-36-8
Purity:98% Package:5 mg
|
| Company Name: |
AlliChem, LLC
|
| Tel: |
301-317-5072 |
| Email: |
sales@allichemllc.com |
| Products Intro: |
|
| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Email: |
enquiry@leancare.co.uk |
| Products Intro: |
|
|
| | MOTRETINIDE Basic information |
| Product Name: | MOTRETINIDE | | Synonyms: | MOTRETINIDE;Tasmaderm;(2E,4E,6E,8E)-N-Ethyl-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetrenamide;N-Ethyl-9-[4-methoxy-2,3,6-trimethylphenyl]-3,7-dimethyl-2,4,6,8-nonatetraenamide;2,4,6,8-Nonatetraenamide, N-ethyl-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-, (2E,4E,6E,8E)- | | CAS: | 56281-36-8 | | MF: | C23H31NO2 | | MW: | 353.5 | | EINECS: | 260-094-6 | | Product Categories: | | | Mol File: | 56281-36-8.mol | 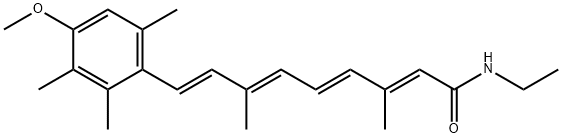 |
| | MOTRETINIDE Chemical Properties |
| | MOTRETINIDE Usage And Synthesis |
| Originator | Tasmaderm,Roche,Switz.,1981 | | Uses | Keratolytic. | | Definition | ChEBI: Motretinide is a retinoid. | | Manufacturing Process | 228 g of 5-(4-methoxy-2,3,6-trimethyl-phenyl)-3-methyl-penta-2,4-diene-1-
triphenylphosphonium bromide are introduced under nitrogen gassing into 910
ml of dimethylformamide and treated with cooling at 5°C to 10°C within 20
minutes with 17.5 g of a suspension of sodium hydride (about 50% by
weight) in mineral oil. The mixture is stirred for 1 hour at about 10°C, then
treated at 5°C to 8°C dropwise with 61.8 g of 3-formylcrotonic acid butyl
ester, heated for 2 hours at 65°C, subsequently introduced into 8 liters of ice�water and, after the addition of 300 g of sodium chloride, thoroughly
extracted with a total of 18 liters of hexane. The extract is washed 5 times
with 1 liter of methanol/water (6:4 parts by volume) each time and 2 times
with 1.5 liters of water each time, dried over sodium sulfate and evaporated
under reduced pressure to leave 9-(4-methoxy-2,3,6-trimethyl-phenyl)-3,7-
dimethyl-nona-2,4,6,8-tetraen-1-oic acid butyl ester, MP 80°C to 81°C as the
residue.
125.8 g of 9-(4-methoxy-2,3,6-trimethyl-phenyl)-3,7-dimethyl-nona-2,4,6,8-
tetraen-1-oic acid butyl ester are introduced into 2,000 ml of absolute ethanol
and treated with a solution of 125.8 g of potassium hydroxide in 195 ml of
water. The mixture is heated to boiling under nitrogen gassing for 30 minutes,
then cooled, introduced into 10 liters of ice-water and, after the addition of
about 240 ml of concentrated hydrochloric acid (pH 2-4), thoroughly extracted
with a total of 9 liters of methylene chloride. The extract is washed with about
6 liters of water to neutrality, dried over calcium chloride and evaporated
under reduced pressure. The residue is taken up in 700 ml of hexane. The
precipitated 9-(4-methoxy-2,3,6-trimethyl-phenyl)-3,7-dimethyl-nona-2,4,6,8-
tetraen-1-oic acid melts at 228°C to 230°C.
28.6 g of 9-(4-methoxy-2,3,6-trimethyl-phenyl)-3,7-dimethyl-nona-2,4,6,8-
tetraen-1-oic acid are introduced into 300 ml of benzene and treated under
nitrogen gassing with 12 g of phosphorus trichloride. The benzene is
subsequently distilled off under reduced pressure. The remaining 9-(4-
methoxy-2,3,6-trimethyl-phenyl)-3,7-dimethyl-nona-2,4,6,8-tetraen-1-oic acid
chloride is dissolved in 1,200 ml of diethyl ether. The solution is added
dropwise at -33°C into 500 ml of ethylamine and stirred for 3 hours. The
reaction mixture is then diluted with 500 ml of diethyl ether and stirred
without cooling for a further 12 hours, the ammonia evaporating. The residue
is dissolved in 10 liters of methylene chloride. The solution is washed 2 times
with 3 liters of water, dried over sodium sulfate and evaporated under reduced
pressure. The remaining N-ethyl-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-
dimethylnona-2,4,6,8-tetraen-1-oic acid amide melts, after recrystallization
from ethanol, at 179°C to 180°C. | | Brand name | Tasmaderm (Hoffmann-LaRoche). | | Therapeutic Function | Antipsoriatic |
| | MOTRETINIDE Preparation Products And Raw materials |
|