|
|
| | DILAZEP DIHYDROCHLORIDE Basic information |
| Product Name: | DILAZEP DIHYDROCHLORIDE | | Synonyms: | DILAZEP DIHYDROCHLORIDE;1,4-BIS[3-(3,4,5-TRIMETHOXYBENZOYLOXY)PROPYL]HOMOPIPERAZINE DIHYDROCHLORIDE;N,N'-BIS[3-(3,4,5-TRIMETHOXY-BENZOYLOXY)PROPYL]HOMOPIPER-AZINE DIHYDROCHLORIDE;1,4-bis(3-(3,4,5-trimethoxybenzoyloxy)-propyl)perhydro-1,4-diazepinedihydroc;astac;astac4898;benzoicacid,3,4,5-trimethoxy-,diesterwithtetrahydro-1h-1,4-diazepine-1,4(5;comelian | | CAS: | 20153-98-4 | | MF: | C31H46Cl2N2O10 | | MW: | 677.61 | | EINECS: | 243-548-8 | | Product Categories: | | | Mol File: | 20153-98-4.mol | 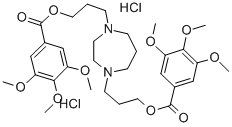 |
| | DILAZEP DIHYDROCHLORIDE Chemical Properties |
| Melting point | 194-198° (monohydrate) | | storage temp. | 2-8°C | | solubility | H2O: 10 mg/mL | | form | powder | | color | white | | Water Solubility | Soluble to 100 mM in water |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 2 | | RTECS | DI0250000 | | Toxicity | LD50 in male mice, male rats (mg/kg): 26.6, 19.1 i.v.; 161, 90.1 i.p.; 3740, >2150 orally (Abel) |
| | DILAZEP DIHYDROCHLORIDE Usage And Synthesis |
| Originator | Cormelian,Asta-Werke,W. Germany,1972 | | Uses | Dilazep Dihydrochloride is a research product for neuroscience. This compound may exhibit neuroprotective properties. Angiotensin converting, adenosine reuptake inhibitor. Antiplatelet agent. Dilazep acts as a cerebral vasodialator. | | Uses | Dilazep dihydrochloride has been used in estrogen receptor (ERα) antagonistic assay. It has also been used as an equilibrative nucleoside transporter inhibitor (ENT 1 and 2) in cancer cell lines. | | Uses | Vasodilatator;Adenosine uptake inhibitor | | Definition | ChEBI: Dilazep dihydrochloride is the dihydrochloride salt of dilazep. It inhibits adenosine uptake, ischemic damage, platelet aggregation, and membrane transport of nucleosides. It has a role as a cardioprotective agent, a platelet aggregation inhibitor and a vasodilator agent. It contains a dilazep(2+). | | Manufacturing Process | 528.8 grams of bis-(3-hydroxypropyl)-ethylene diamine [K. Schlgl and R.
Schlgl, Monatschefte der Chemie 95 (1964) page 935] are dissolved in a
mixture of 1,500 cc of anhydrous ethyl alcohol and 1,250 grams of
triethylamine. 520 grams of 1,3-chlorobromopropane are added thereto
dropwise over a period of about 3 hours while stirring and heating the
reaction mixture in an oil bath of 50°C. After completion of the addition, the
oil bath is heated to 60°C for 20 minutes while stirring of the reaction mixture
is continued. With increasing reaction time, triethylamine hydrochloride is
precipitated. After completion of the reaction, the mixture is allowed to cool to
room temperature.
Triethylamine hydrochloride is separated by filtration and the filter cake is
washed with 100 cc of anhydrous ethyl alcohol. The alcohol and the excess of
triethylamine is distilled off in a vacuum of a water pump. The residue
represents a light-yellowish brown viscous oil which is extracted 3 times with
500 cc of anhydrous benzene each time with stirring at 40° to 60°C. The
benzene is distilled off on a water bath at 60°C. Thus, an oil is obtained which
solidifies to a hard mass after some hours. This mass is crushed and dried over P2O5 in an exsiccator. The compound represents N,N'-bis-(3-
hydroxypropyl)homopiperazine. Yield: 128.5 grams. FP: 46°-47°C; BP0.02mm:
141°-142°C.
21.6 grams of N,N'-bis-(3-hydroxypropyl)homopiperazine obtained as
described and 63.8 grams of 3,4,5-trimethoxy benzoic acid chloride are
dissolved in 600 parts by volume of anhydrous chloroform. The solution is
heated to boiling for 5 hours. Thereafter, chloroform is distilled off in a
vacuum. The residue is dissolved in water and the aqueous solution is washed
with ether. Thereafter, the aqueous phase is rendered alkaline by the addition
of soda lye and the separated oil base is extracted with ether. The ethereal
solution is dried over Na2SO4. Ether is separated in a vacuum and the highly
viscous residue is dissolved in 150 parts by volume of ethyl alcohol. The
calculated equivalent amount of ethereal HCl is added thereto.
The soon crystallizing dihydrochloride is separated by filtration, dried and
recrystallized from 120 parts by volume of ethanol. Thus, after drying for 3
days over P2O5, 40-50 grams (66-70% of the theoretical) of N,N'-bis-[(3,4,5-
trimethoxy benzoloxy)propyl] homopiperazine dihydrochloride containing 1
mol of water of crystallization is obtained. This product has a melting point at
194°-198°C. | | Therapeutic Function | Coronary vasodilator | | General Description | Dilazep comprises central cyclic?diamine and phenyl rings connected via alkyl linkers. | | Biochem/physiol Actions | Dilazep is a equilibrative nucleoside transporter 1 (ENTs) inhibitor. It is an antianginal drug and an effective coronary vasodilator. Dilazep elicits anti-albuminuric effects and is an anti-platelet agent. It is a potent adenosine uptake inhibitor and suppresses effects of ischemia. | | storage | Store at RT |
| | DILAZEP DIHYDROCHLORIDE Preparation Products And Raw materials |
|