| Company Name: |
Tcichem, Inc.
|
| Tel: |
13918644899 |
| Email: |
tianchong009@hotmail.com |
| Products Intro: |
Product Name:2-(dibutylcarbamoyloxy)-N-ethyl-N,N-dimethylethanaminium chloride
CAS:532-49-0
Purity:95% HPLC Package:1G;5G;10G;25G;50G;100G;1KG
|
|
| | Dibutoline Basic information |
| Product Name: | Dibutoline | | Synonyms: | (2-Dibutylcarbamyloxyethyl)dimethylethylammonium sulfate;Ammonium, ethyl(2-hydroxyethyl)dimethyl-, dibutylcarbamate (ester), sulfate (2:1);Ammonium, ethyl(2-hydroxyethyl)dimethyl-, sulfate (salt), bis(dibutylcarbamate);Bis[(dibutylcarbamate) of ethyl(2-hydroxyethyl)dimethylammonium] sulfate;Dibuline sulfate;Dibulinesulfat;Di-N-butyl-carbamylcholine sulphate;2-(dibutylcarbamoyloxy)ethyl-ethyl-dimethylazanium | | CAS: | 532-49-0 | | MF: | 2C15H33N2O2.O4S | | MW: | 642.937 | | EINECS: | | | Product Categories: | | | Mol File: | 532-49-0.mol | 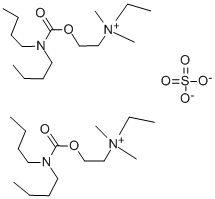 |
| | Dibutoline Chemical Properties |
| | Dibutoline Usage And Synthesis |
| Originator | Dibuline Sulfate,MSD ,US,1952 | | Manufacturing Process | About 55.5 g of β-chloroethyl-di-n-butylcarbamate and about 22.6 g of
dimethylamine are placed in a container, firmly sealed, and heated at about
95°C for about 16 hours. To the resulting crude mixture is added ethyl ether
and the mixture filtered to remove dimethylamine hydrochloride formed
during the course of the reaction. The ethereal solution is then extracted with
12 N hydrochloric acid. Under a fresh layer of ether and at a temperature
under 10°C the aqueous acid extract is first neutralized with sodium carbonate
and then made strongly alkaline with sodium hydroxide. The supernatant
ethereal solution is then separated and dried over potassium hydroxide. The
ethereal solution is finally concentrated and the residue obtained is fractionally
distilled under vacuum. The β-dimethylaminoethyl-di-n-butylcarbamate is
found to distill undecomposed at about 128°C to 130°C under approximately
2 mm pressure.
A mixture of about 100 g of β-dimethylaminoethyl-di-n-butylcarbamate and
about 188 cc of ethyl iodide is held at about 25°C for two hours. The
temperature is kept about 25°C by occasional cooling in an ice bath during
the first half hour. About 1,600 cc of anhydrous ethyl ether is then added
causing the precipitation of a dense white product. After standing for about 16
hours at 0°C the product is filtered off, washed thoroughly with anhydrous
ether, and dried under diminished pressure at room temperature over sulfuric
acid. The β-(dimethyl ethyl ammonium iodide)-ethyl-di-n-butylcarbamate thus
obtained is a white crystalline powder, slightly hygroscopic with a melting
point of about 76°C to 77°C.
A mixture of about 150 g of β-(dimethyl ethyl ammonium iodide)-ethyl-di-nbutylcarbamate, 90 g of silver sulfate, 750 cc of water and 750 cc of ethanol
is stirred at about 30°C for approximately 45 minutes. The silver iodide
formed is removed and the excess silver remaining in solution is removed by
bubbling in hydrogen sulfide for five minutes followed by filtration to remove
the precipitated silver sulfide. The filtrate is concentrated to a thick syrup
under vacuum and about one liter of benzene is added which is distilled off
with stirring to atmospheric pressure to remove the last traces of water. The
residual benzene is removed under vacuum and the product granulated by
stirring with one liter of anhydrous ether for two hours. The product is
removed, washed with anhydrous ether, and dried under diminished pressure
over phosphorous pentoxide at 25°C. The β-(dimethyl ethyl ammonium
sulfate)-ethyl-di-n-butylcarbamate thus obtained is a very hygroscopic white
solid having a melting point of about 166°C with decomposition. | | Therapeutic Function | Anticholinergic (ophthalmic) |
| | Dibutoline Preparation Products And Raw materials |
|