| Company Name: |
Molsyns Research
|
| Tel: |
+91-9586858886 +91-9586858886 |
| Email: |
info@molsyns.com |
| Products Intro: |
Product Name:Budesonide EP Impurity-H
CAS:313474-58-7
Purity:98% Package:25mg; 50mg;100mg and 1 g
|
|
| | 9,11-Anhydrobudesonide Basic information |
| Product Name: | 9,11-Anhydrobudesonide | | Synonyms: | 9,11-Anhydrobudesonide;Budesonide EP Impurity H;16α,17-[(1RS)-butylidenebis(oxy)]-21-hydroxypregna1,4,9(11)-triene-3,20-dione;9,11-Anhydrobudesonide\n\n(Mixture of Diastereomers);Budesonide Impurity 8(Budesonide EP Impurity H);Budesonide iMpurit H;Budesonide EP Impurity H (Mixture of Diastereomers);Budesonide EP Imp H | | CAS: | 313474-58-7 | | MF: | C25H32O5 | | MW: | 412.52 | | EINECS: | | | Product Categories: | | | Mol File: | 313474-58-7.mol | 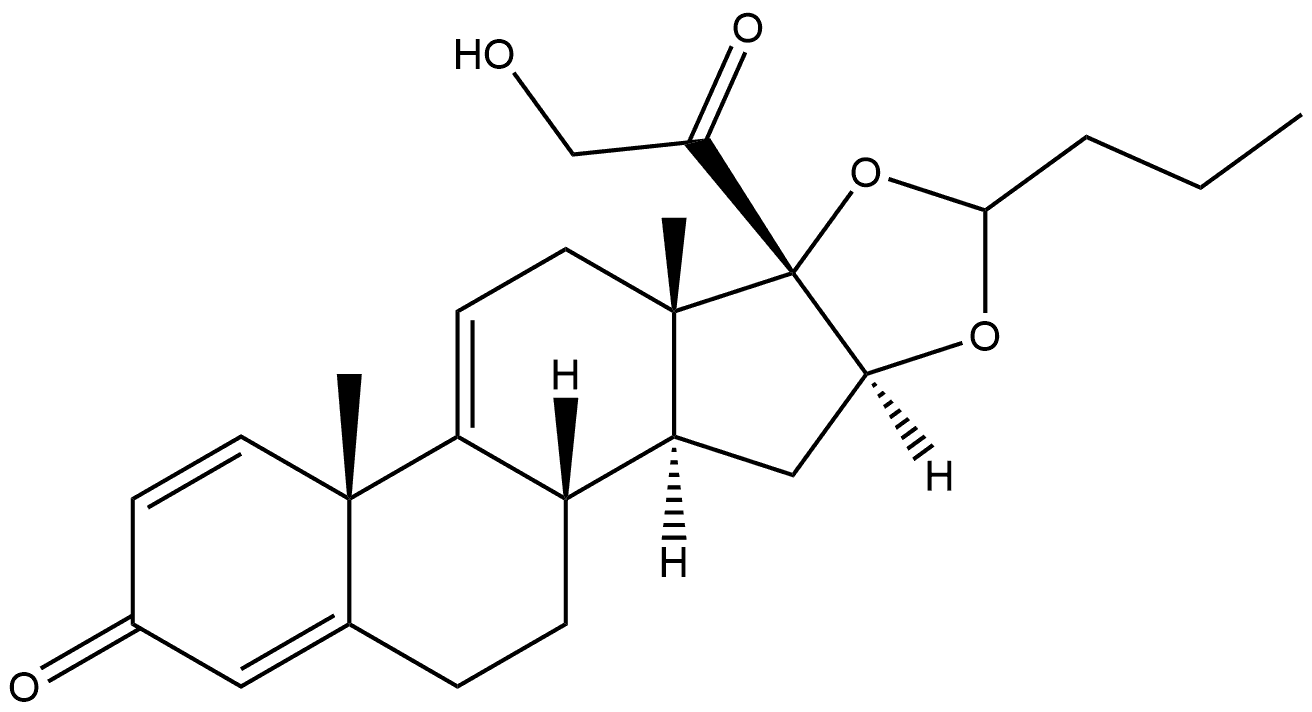 |
| | 9,11-Anhydrobudesonide Chemical Properties |
| Boiling point | 577.6±50.0 °C(Predicted) | | density | 1.24±0.1 g/cm3(Predicted) | | solubility | Chloroform (Slightly), Methanol (Slightly) | | pka | 12.85±0.10(Predicted) | | form | Solid | | color | White to Off-White |
| | 9,11-Anhydrobudesonide Usage And Synthesis |
| Uses | 9,11-Anhydrobudesonide (Budesonide EP Impurity H) is an impurity of Budesonide (B689460), a non-halogenated glucocorticoid intended for the local treatment of lung disease. |
| | 9,11-Anhydrobudesonide Preparation Products And Raw materials |
|