|
|
| | 16α-Butyloxyprednisolone (Budenoside IMpurity) Basic information | | Uses |
| Product Name: | 16α-Butyloxyprednisolone (Budenoside IMpurity) | | Synonyms: | 16α-Butyloxyprednisolone (Budenoside IMpurity);Budesonide Impurity I;Budenoside Impurity I(EP);Budesonide Impurity 9(Budesonide EP Impurity I);11β,17,21-trihydroxy-3,20-dioxopregna-1,4-dien-16α-yl butanoate;Budenoside EP Impurity I;Budesonide EP Imp I;16alpha-Butyloxyprednisolone | | CAS: | 113930-13-5 | | MF: | C25H34O7 | | MW: | 446.53 | | EINECS: | | | Product Categories: | | | Mol File: | 113930-13-5.mol | 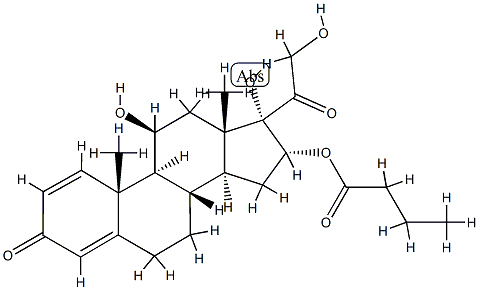 |
| | 16α-Butyloxyprednisolone (Budenoside IMpurity) Chemical Properties |
| Melting point | >115°C (dec.) | | Boiling point | 617.9±55.0 °C(Predicted) | | density | 1.30±0.1 g/cm3(Predicted) | | storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere | | solubility | Chloroform (Slightly, Sonicated), DMSO (Slightly), Methanol (Slightly) | | pka | 11.39±0.70(Predicted) | | form | Solid | | color | White to Off-White | | Stability: | Hygroscopic |
| | 16α-Butyloxyprednisolone (Budenoside IMpurity) Usage And Synthesis |
| Uses | 16α-Butyloxyprednisolone (Budesonide EP Impurity I) is an impurity of Budesonide, a non-halogenated glucocorticoid intended for the local treatment of lung disease. | | Uses | 16α-Butyloxyprednisolone (Budesonide EP Impurity I) is an impurity of Budesonide (B689460), a non-halogenated glucocorticoid intended for the local treatment of lung disease. |
| | 16α-Butyloxyprednisolone (Budenoside IMpurity) Preparation Products And Raw materials |
|