- Methylprednisolone
-

- $35.00/ kg
-
2024-03-16
- CAS:83-43-2
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 200tons/year
- Methylprednisolone
-

- $0.00 / 1KG
-
2024-03-16
- CAS:83-43-2
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
- Methylprednisolone
-

- $0.00 / 1kg
-
2023-11-27
- CAS:83-43-2
- Min. Order: 1kg
- Purity: 99% min
- Supply Ability: 20 tons
|
| | Methylprednisolone Basic information |
| Product Name: | Methylprednisolone | | Synonyms: | 20-dione,11-beta,17,21-trihydroxy-6-alpha-methyl-pregna-4-diene-3;20-dione,11beta,17,21-trihydroxy-6alpha-methyl-pregna-4-diene-3;20-dione,6-alpha-methyl-11-beta-17,21-trihydroxy-pregna-4-diene-3;6alpha-methyl-11beta,17alpha,21-triol-1,4-pregnadiene-3,20-dione;artisone-wyeth;Besonia;delta(1)-6alpha-methylhydrocortisone;Suprametil | | CAS: | 83-43-2 | | MF: | C22H30O5 | | MW: | 374.47 | | EINECS: | 201-476-4 | | Product Categories: | Intermediates & Fine Chemicals;Hormone Drugs;Miscellaneous Biochemicals;Biochemistry;Hydroxyketosteroids;Steroids;MEDROL;Steroid and Hormone;API;Pharmaceuticals;83-43-2 | | Mol File: | 83-43-2.mol | 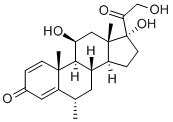 |
| | Methylprednisolone Chemical Properties |
| Melting point | 228-237°C (dec.) | | alpha | D20 +83° (dioxane) | | Boiling point | 423.47°C (rough estimate) | | density | 1.0868 (rough estimate) | | refractive index | 82 ° (C=1, Dioxane) | | storage temp. | 0-6°C | | solubility | chloroform/methanol (9:1): 50 mg/mL, clear, faintly yellow | | pka | 12.46±0.70(Predicted) | | color | White to Off-White | | Water Solubility | 0.12g/L(25 ºC) | | Merck | 14,6111 | | BRN | 2340300 | | BCS Class | 2 or 4? | | InChIKey | VHRSUDSXCMQTMA-PJHHCJLFSA-N | | CAS DataBase Reference | 83-43-2(CAS DataBase Reference) | | NIST Chemistry Reference | 1,4-Pregnadiene-3,20-dione, 11beta,17alpha,21-trihydroxy-6alpha-methyl-(83-43-2) | | EPA Substance Registry System | Pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-6-methyl-, (6.alpha.,11.beta.)- (83-43-2) |
| | Methylprednisolone Usage And Synthesis |
| Chemical Properties | White to Off-White Crystalline Powder | | Originator | Medrol,Upjohn,US,1957 | | Uses | A glucocorticoid which displays anti-inflamatory and antioxidant properties and attenuates apoptosis in oligodendrocytes after injury. 6α-Methyl Prednisolone is neuroprotective. | | Definition | ChEBI: The 6alpha-stereoisomer of 6-methylprednisolone. | | Manufacturing Process | The following process description is taken from US Patent 2,897,218. Six 100-
ml portions of a medium in 250-ml Erlenmeyer flasks containing 1% glucose,
2% corn steep liquor (60% solids) and tap water was adjusted to a pH of 4.9.
This medium was sterilized for 45 minutes at 15 psi pressure and inoculated
with a one to two day growth of Septomyxa affinis ATCC 6737. The
Erlenmeyer flasks were shaken at room temperature at about 24°C for a
period of 3 days.
At the end of this period, this 600-ml volume was used as an inoculum for ten
liters of the same glucose-corn steep liquor medium which in addition
contained 10 ml of an antifoam (a mixture of lard oil and octadecanol). The
fermentor was placed into the water bath, adjusted to 28°C, and the contents
stirred (300 rpm) and aerated (0.5 liter air/10 liters beer). After 17 hours of
incubation, when a good growth developed and the acidity rose to pH 6.7, 2 g
of 6α-methylhydrocortisone plus 1 g of 3-ketobisnor-4-cholen-22-al, dissolved
in 115 ml of dimethylformamide, was added and the incubation (conversion)
carried out at the same temperature and aeration for 24 hours (final pH 7.9).
The mycelium (56 g dry weight) was filtered off and the steroidal material was
extracted with methylene chloride, the methylene extracts evaporated to
dryness, and the resulting residue chromatographed over a Florisil column.
The column was packed with 200 g of Florisil and was developed with five
400-ml fractions each of methylene chloride, Skellysolve B-acetone mixtures
of 9:1, 8:2, 7:3, 1:1, and methanol. The fraction eluted with Skellysolve Bacetone (7:3) weighed 1.545 g and on recrystallization from acetone gave, in
three crops, 928 mg of product of MP 210° to 235°C. The sample prepared for
analysis melted at 245° to 247°C. | | Brand name | Medrol (Pharmacia & Upjohn). | | Therapeutic Function | Glucocorticoid | | General Description | Methylprednisolone, 11β,17,21-trihydroxy-6α-methyl-1,4-pregnadiene-3,20-dione, isavailable unmodified or as ester derivatives.
Methylprednisolone acetate, USP
Methylprednisolone sodium succinate, USP. | | Biological Activity | methylprednisolone is a synthetic glucocorticoid receptor agonist, used to achieve prompt suppression of inflammation.cllinical trials: in patients with acute spinal cord injury diagnosed in national acute spinal cord injury study (nascis) centers within 8 hours of injury, methylprednisolone treatment for 48 hours improved motor recovery at 6 weeks (p= 0.09) and 6 months (p= 0.07) after injury[6]. in patients with acute spinal-cord injury, methylprednisolone (30 mg/kg) followed by infusion at 5.4 mg/kg/hour for 23 hours improved neurologic recovery. among patients treated with methylprednisolone (30 mg/kg), mortality at 14 days was significantly increasedsecondary infection[7]. methylprednisolone has entered | | Mechanism of action | Methylprednisolone is an analog of prednisolone that exhibits a more prolonged effect
than prednisolone and cortisone; it has practically no mineralocorticosteroid activity and
is better tolerated. | | Clinical Use | Adding a 6α-methyl group to prednisolone increases the glucocorticoid activity and effectively
abolishes mineralocorticoid action. It has fivefold the glucocorticoid activity of hydrocortisone
(prednisolone has fourfold the glucocorticoid activity) and none of its mineralocorticoid
properties. It is used almost exclusively as a systemic product and is available as the free alcohol
for oral administration and as various esters. | | Safety Profile | Moderately toxic by
intraperitoneal route. A steroid hormone.
Human systemic effects include arrhythmias,
blood pressure lowering, heart rate changes,
increased body temperature, pulse rate
increase, respiratory depression. When heated to decomposition it emits acrid
smoke and irritating fumes. | | Synthesis | Methylprednisolone, 11|?,17|á,21-trihydroxy-6|á-methylpregna-1,
4-dien-3,20-dione (27.1.38), differs from prednisolone in the presence of a methyl group at
position C6 of the steroid skeleton of the molecule. This seemingly simple difference in structure
requires a different approach to synthesis. It is synthesized from hydrocortisone (27.1.8),
the carbonyl group of which initially undergoes ketalization by ethylene glycol in the presence
of traces of acid, during which the double bond at position C4¨C C5 is shifted to position C5¨C C6,
giving the diethyleneketal 27.1.34. The product is oxidized to an epoxide (27.1.35) using perbenzoic
acid. Next, the resulting epoxide is reacted with methylmagnesium bromide, and subsequent
removal of the ketal protection by hydrogen reduction gives the 5-hydroxy-6-methyl
derivative of dihydrocortisone 27.1.36. The resulting |?-hydroxyketone is dehydrated using an
alkaline, and then the resulting 6|á-methylcortisone (27.1.37) undergoes microbiological
dehydration at position C1¨CC2, giving the desired methylprednisolone (27.1.38). 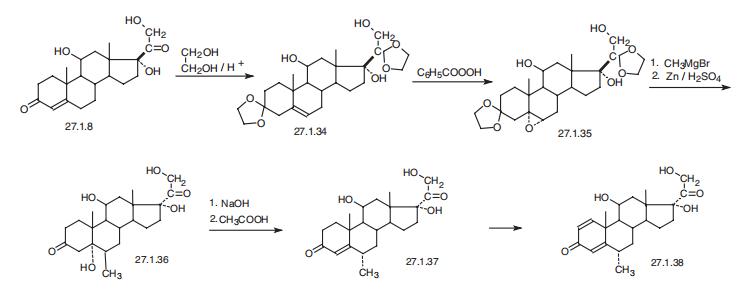
| | in vitro | methylprednisolone (2-10 mg/kg) significantly inhibited tnf production. high doses of methylprednisolone (50 mg/kg) increased lps-induced il-10 levels. methylprednisolone (0.01-100 μg/ml) increased the biosynthesis of il-10 in lps-activated mouse peritoneal macrophages [1]. in wg patients and controls, methylprednisolone (mp) down-regulated the spontaneous and the staphylococcal enterotoxin b (seb)-induced release of chemokines from peripheral blood mononuclear cells (pbmc)[2]. treatment with 0.25 mm methylprednisolone inhibited acantholysis in skin cultures directly [3]. | | in vivo | methylprednisolone (30 mg/kg, i.v.) given immediately after sci reduced tnf-α expression by 55% (p<0.01) and nf-kb binding activity. methylprednisolone suppressed the post-traumatic inflammatory activity caused by tnf-alpha-nf-kb cascade[4]. intravenously administration of mp (30 mg/kg) reduced the number of ed1-positive cells by 82% in the rostral cord stump and 66% in the caudal stump. in the adult rat, mp administration shortly after spinal cord transection resulted in a long-term reduction of ed1-positive cells and spinal tissue loss, reduced dieback of vestibule spinal fibres, and a transient sprouting of vestibule spinal fibres near the lesion at 1 and 2 weeks post-lesion. mp treatment also significantly reduced the tissue loss in both cord stumps at 2, 4 and 8 week post-injury[5]. | | Drug interactions | Metabolism in the liver occurs primarily via the CYP3A4
enzyme to inactive metabolites, which are excreted in the
urine Aldesleukin: avoid concomitant use.
Antibacterials: metabolism accelerated by rifampicin;
metabolism inhibited by erythromycin and possibly
clarithromycin; concentration of isoniazid possibly
reduced.
Anticoagulants: efficacy of coumarins and
phenindione may be altered.
Antiepileptics: metabolism accelerated by
carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone.
Antifungals: increased risk of hypokalaemia with
amphotericin - avoid; metabolism inhibited by
ketoconazole and possibly itraconazole.
Antivirals: concentration possibly increased by
ritonavir.
Ciclosporin: rare reports of convulsions in patients
on ciclosporin and high-dose corticosteroids;
levels of ciclosporin increased with high dose
methylprednisolone.
Cobicistat: concentration possibly increased by
cobicistat.
Diuretics: enhanced hypokalaemic effects of
acetazolamide, loop diuretics and thiazide diuretics.
Vaccines: high dose corticosteroids can impair
immune response to vaccines; avoid with live
vaccines. | | Metabolism | Metabolism in the liver occurs primarily via the CYP3A4
enzyme to inactive metabolites, which are excreted in the
urine | | storage | Store at +4°C | | Purification Methods | Recrystallise medrol from EtOAc. The UV has max at in 95% EtOH 243nm ( 14,875). The 21-acetoxy derivative has m 205-208o (from EtOAc), and [�] D +95o ( c 1, CHCl3). [Spero et al. J Am Chem Soc 78 6213 1956; Fried et al. J Am Chem Soc 81 1235 1959; 1H NMR: Slomp & McGarvey J Am Chem Soc 81 2200 1959, Beilstein 8 IV 3498.] | | references | [1] marchant a, amraoui z, gueydan c, et al. methylprednisolone differentially regulates il‐10 and tumour necrosis factor (tnf) production during murine endotoxaemia[j]. clinical & experimental immunology, 1996, 106(1): 91-96.
[2] torheim e a, yndestad a, bjerkeli v, et al. increased expression of chemokines in patients with wegener's granulomatosis modulating effects of methylprednisolone in vitro[j]. clinical & experimental immunology, 2005, 140(2): 376-383.
[3] swanson d l, dahl m v. methylprednisolone inhibits pemphigus acantholysis in skin cultures[j]. journal of investigative dermatology, 1983, 81(3): 258-260.
[4] xu j, fan g, chen s, et al. methylprednisolone inhibition of tnf-α expression and nf-kb activation after spinal cord injury in rats[j]. molecular brain research, 1998, 59(2): 135-142.
[5] oudega m, vargas c g, weber a b, et al. [j]. european journal of neuroscience, 1999, 11(7): 2453-2464.long‐term effects of methylprednisolone following transection of adult rat spinal cord
[6] bracken m b, shepard m j, holford t r, et al. administration of methylprednisolone for 24 or 48 hours or tirilazadmesylate for 48 hours in the treatment of acute spinal cord injury: results of the third national acute spinal cord injury randomized controlled trial[j]. jama, 1997, 277(20): 1597-1604.
[7] bracken m b, shepard m j, collins w f, et al. a randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the second national acute spinal cord injury study[j]. new england journal of medicine, 1990, 322(20): 1405-1411. |
| | Methylprednisolone Preparation Products And Raw materials |
|