| | Pseudoephedrine Basic information |
| Product Name: | Pseudoephedrine | | Synonyms: | S,S(+)-Pseudoephedrine solution;(+)-pseudoephedrin;(+)-psi-ephedrin;(alpha-s)-benzenemethano;[S-(R*,R*)]-alpha-[1-(methylamino)ethyl]benzenemethanol;2-(Methylamino)-1-phenyl-1-propanol;alpha-(1-(methylamino)ethyl)-,(s-(r*,r*))-benzenemethano;alpha-[1-(methylamino)ethyl]-,[s-(theta,theta)]-benzenemethano | | CAS: | 90-82-4 | | MF: | C10H15NO | | MW: | 165.23 | | EINECS: | 202-018-6 | | Product Categories: | chemical research;pharmaceutical intermediate | | Mol File: | 90-82-4.mol | 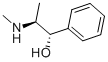 |
| | Pseudoephedrine Chemical Properties |
| Melting point | 118-120 °C | | alpha | 52 º (c=0.6, EtOH) | | Boiling point | 293.09°C (rough estimate) | | density | 1.0203 (rough estimate) | | vapor pressure | 0.016-0.032Pa at 20-25℃ | | refractive index | 1.5200 (estimate) | | Fp | 9℃ | | storage temp. | -20°C | | solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; Ethanol: 30 mg/ml; PBS (pH 7.2): 5 mg/ml | | form | Crystalline | | pka | pKa 9.73(H2O,t=25±0.5,I=0.01)(Approximate) | | optical activity | [α]20/D +52°, c = 0.6 in ethanol | | Water Solubility | <0.5g/L(er) | | Merck | 13,8007 | | BRN | 2414132 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. May discolour upon exposure to light. | | LogP | 0.89 at 25℃ | | CAS DataBase Reference | 90-82-4(CAS DataBase Reference) | | NIST Chemistry Reference | Pseudoephedrine, (+)-(90-82-4) | | EPA Substance Registry System | Benzenemethanol, .alpha.-[(1S)-1-(methylamino)ethyl]-, (.alpha.S)- (90-82-4) |
| | Pseudoephedrine Usage And Synthesis |
| Description | Pseudoephedrine is a stereoisomer of ephedrine, in the drug
class of sympathomimetics. It occurs naturally in plants of the
genus Ephedra. Pseudoephedrine is a mixed-acting decongestant,
which activates α- and β-adrenergic receptors directly by
binding to the receptor itself, and indirectly by causing
norepinephrine release in synaptic nerve terminals.
Pseudoephedrine is also used illicitly in the production of
methamphetamine. In the United States, two recent acts of legislation
– the Combat Methamphetamine Epidemic Act of 2005
and the Methamphetamine Production Prevention Act of 2008–
have created stringent regulation on the sale of pseudoephedrine
without a prescription. Pseudoephedrine-containing products
were moved behind the counter, only to be sold by the
pharmacist using their professional judgment and discretion.
Limitations on the quantity of pseudoephedrine that could be
purchased at one time and over a period of timewere enacted, and
strict record keeping was required. | | Description | (1S,2S)-(+)-Pseudoephedrine (Item No. 14209) is an analytical reference standard that is categorized as an amphetamine. (1S,2S)-(+)-Pseudoephedrine is a precursor in the synthesis of methamphetamine (Item Nos. 13997 | 13998 | ISO60168). This product is intended for research and forensic applications. | | Chemical Properties | white crystals | | Uses | Pseudoephedrine is an orally active sympathomimetic amine
that is used as a nasal decongestant. It exerts its decongestant
action by acting directly on a-adrenergic receptors in the
respiratory tract mucosa producing vasoconstriction resulting
in shrinkage of swollen nasal mucous membranes, reduction of
tissue hyperemia, edema, and nasal congestion, and an increase
in nasal airway patency. Drainage of sinus secretions is
increased and obstructed eustachian ostia may be opened.
Relaxation of bronchial smooth muscle by stimulation of
b-adrenergic receptors may also occur. | | Definition | ChEBI: A member of the class of the class of phenylethanolamines that is (1S)-2-(methylamino)-1-phenylethan-1-ol in which the pro-S hydrogen at position 2 is replaced by a methyl group. | | Brand name | Efidac (ALZA); Novafed (Sanofi Aventis);
Sudafed (GlaxoSmithKline); Sudafed (Warner Lambert). | | General Description | (Sudafed, Afrinol, Drixoral) isthe (S,S) diastereoisomer of ephedrine. Whereas ephedrinehas a mixed mechanism of action, L-(+)-pseudoephedrineacts mostly by an indirect mechanism and has virtually nodirect activity. The structural basis for this difference inmechanism is the stereochemistry of the carbon atom possessingthe β-OH group. In pseudoephedrine, this carbon atompossesses the (S) configuration, the wrong stereochemistryat this center for a direct-acting effect at adrenoceptors.Although it crosses the BBB (log P=1.05, pKa=9.38),L-(+)-pseudoephedrine’s lack of direct activity affords fewerCNS effects than does ephedrine. It is a naturally occurringalkaloid from the Ephedra species. This agent is found inmany OTC nasal decongestant and cold medications.Although it is less prone to increase blood pressure thanephedrine, it should be used with caution in hypertensiveindividuals, and it should not be used in combination withMAO inhibitors. | | Contact allergens | This sympathomimetic a-adrenergic agonist is found
in plants of the genus Ephedra (Ephedraceae) and is
systemically used as a nasal decongestant. It can induce
drug skin reactions such as acute generalized exanthematic
pustulosis or generalized eczema. | | Environmental Fate | Through use as a decongestant and production, release to
the environment may result from various waste streams. Pseudoephedrine is also found in plants in the genus Ephedra
(Ephedraceae) otherwise known as Ma Huang. It has a vapor
pressure of 8.3×10�4 mm Hg at 25 °�C and if released into air
it will exist both as vapor and in particulate phase in the
atmosphere. Vapor-phase pseudoephedrine will be degraded
by reactions with hydroxyl radicals, which are photochemically
produced. The half-life for this reaction is estimated at 4 h.
Particulate-phase pseudoephedrine will be removed from the
atmosphere by wet and dry deposition. Pseudoephedrine is not
susceptible to direct photolysis by sunlight.
Based upon an estimated Koc of 73, pseudoephedrine is
expected to have a high mobility in soil. The pKa of 10.25
indicates that it will exist primarily in the cation form in the
environment and it will absorb more strongly to soil containing
clay or organic carbon. | | Purification Methods | Crystallise the amine from dry diethyl ether, or from water and dry it in a vacuum desiccator. [Beilstein 13 IV 1878.] | | Toxicity evaluation | Pseudoephedrine is a weak base (pKa, 9.4) that stimulates both
α- and β-adrenergic receptors, as well as causing the release of
neuronal norepinephrine. This mixed α/β adrenergic stimulation
produces both hypertension and tachycardia, as opposed
to the hypertension with reflex bradycardia seen with selective
a agonists. |
| | Pseudoephedrine Preparation Products And Raw materials |
|