| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Email: |
enquiry@leancare.co.uk |
| Products Intro: |
|
| Company Name: |
2A PharmaChem USA
|
| Tel: |
(630) 737-0988 |
| Email: |
sales@2apharmachem.com |
| Products Intro: |
|
|
| | PHOSPHONIUM IODIDE Basic information |
| Product Name: | PHOSPHONIUM IODIDE | | Synonyms: | iodinephosphide;jodphosphonium;PHOSPHONIUM IODIDE;Tetrahydridephosphorus iodide;iodophosphorane;Phosphonium iodide ((PH4)I) (9CI) | | CAS: | 12125-09-6 | | MF: | H4IP | | MW: | 159.89 | | EINECS: | 235-189-0 | | Product Categories: | | | Mol File: | 12125-09-6.mol | 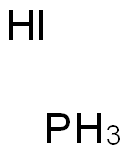 |
| | PHOSPHONIUM IODIDE Chemical Properties |
| Melting point | 18.5° under its own vapor pressure | | Boiling point | 62.5°C (estimate) | | density | 2.860 | | solubility | reacts with H2O, ethanol | | form | colorless tetragonal crystals | | color | colorless tetragonal crystals, crystalline | | Water Solubility | decomposed by H2O, alcohol, evolving phosphine gas [HAW93] |
| | PHOSPHONIUM IODIDE Usage And Synthesis |
| Chemical Properties | large, transparent, colorless crystal(s); tetr; sublimes at room temp; decomposes to PH3 and HI when heated or in presence of alcohol or water; rapid heating causes detonation [MER06] | | Physical properties | Colorless tetragonal crystal; deliquesces; density 2.86 g/cm3; sublimes at ordinary temperatures; vapor pressure 50 torr at 20°C, 760 torr at 61.8°C; melts at 18.5°C under its own vapor pressure; boils at 80°C; decomposes in water; soluble in acids and alkalies with decomposition; decomposes in ethanol. | | Uses | In the laboratory preparation of phosphine. | | Preparation | Phosphonium iodide may be prepared by the action of phosphine with dry hydrogen iodide:
PH3 + HI → PH4I
Solid phosphonium iodide may be produced in the laboratory by slowly and very cautiously adding water to an intimate mixture of white phosphorus and iodine. Also, this phosphorus-iodine mixture may be obtained by mixing solutions in carbon disulfide and carefully evaporating the solvent in a stream of inert gas:
P4 + I + H2O → PH4I + H3PO4
2P + I2 + 4H2O → PH4I + H3PO4 + HI
Also, the compound can be prepared by hydrolysis of a mixture of diphosphorus tetraiodide and white phosphorus:
P2I4 + P + H2O → PH4I + H3PO4. | | Hazard | Phosphonium iodide detonates on rapid heating. It dissociates by water, alcohol or heat, evolving toxic phosphine gas. |
| | PHOSPHONIUM IODIDE Preparation Products And Raw materials |
|